Published: August 2022
Library Resources
- Written by: Rashid K. Sayyid, MD MSc and Zachary Klaassen, MD,MSc
- References:
- Weiner AB, Siebert AL, Fenton SE, et al. First-line Systemic Treatment of Recurrent Prostate Cancer After Primary or Salvage Local Therapy: A Systematic Review of the Literature. Eur Urol Oncol. 2022.
- Cancer Stat Facts: Prostate Cancer. National Cancer Institute. Available at https://seer.cancer.gov/statfacts/html/prost.html. Accessed: Nov 14, 2022
- Deek MP, Van der Eecken K, Phillips R, et al. The mutational landscape of metastatic castration-sensitive prostate cancer: the spectrum theory revisited. Eur Urol. 2021;80:632-640
- Stopsack KH, Nandakumar S, Wimber AG, et al. Oncogenic genomic alterations, clinical phenotypes, and outcomes in metastatic castration-sensitive prostate cancer. Clin Cancer Res. 2020;26:3230-3238.
- Sweeney CJ, Chen Y, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N ENgl J Med. 2015;373:737-746.
- Fizai K, Foulon S, Carles J, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet 2022;399(10336):1695-1707.
- Smith MR, Hussain M, Saad F, et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med. 2022;386(12):1132-1142.
- Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med. 2019;381(2):121-131.
- Hoyle AP, Ali A, James ND, et al. Abiraterone in “High-” and “Low-risk” Metastatic Hormone-sensitive Prostate Cancer. Eur Urol. 2018;76(6):719-728.
- James ND, de Bono JS, Spears MR, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med. 2017;377:338-351.
- Chi KN, Chowdhury S, Bjartell A, et al. Apalutamide in Patients With Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J Clin Oncol. 2021;39(2):2294-2303.
- Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol. 2019;37(32):2974-2986.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353-2366.
- Boeve LMS, Hulshof MCCM, Vis AN, et al. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. Eur Urol. 2019;75(3):410-418.
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: A prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36(5):446-453.
- Phillips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020;6(5):650-659.
- Deek MP, van der Eecken K, Sutera P, et al. Long-Term Outcomes and Genetic Predictors of Response to Metastasis-Directed Therapy Versus Observation in Oligometastatic Prostate Cancer: Analysis of STOMP and ORIOLE Trials. J Clin Oncol. 2022;JCO2200644.
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185):2051-2058.
The Current Landscape of PSMA PET Imaging in Prostate Cancer: Evolution of Next Generation Imaging & PSMA PET for Primary Tumor Evaluation
Imaging plays a significant role in the diagnosis and management of prostate cancer. While transrectal ultrasound and, subsequently, multiparametric magnetic resonance imaging (mpMRI) have become well-established modalities in the initial diagnosis of prostate cancer, numerous techniques for the distant staging of prostate cancer have all suffered from significant limitations.
- Written by: Rashid Sayyid, MD MSc, & Zachary Klaassen, MD MSc
- References:
- Macedo F, Ladeira K, Pinho F, et al. Bone metastases: an overview. Oncol Rev. 2017;11(1):321.
- Heindel W, Gübitz R, Vieth V, et al. The diagnostic imaging of bone metastases. Dtsch Arztebl Int. 2014;111(44):741-7.
- Jadvar H. Is There Utility for FDG PET in Prosate Cancer? Semin Nucl Med. 2016;46(6):502-506.
- Picchio M, Briganti A, Fanti S, et al. The role of choline positron emission tomography/computed tomography in the management of patients with prostate-specific antigen progression after radical treatment of prostate cancer. Eur Urol 2011;59:51-60.
- Krause BJ, Souvatzoglou M, Tuncel M, et al. The detection rate of [11C]choline-PET/CT depends on the serum PSA-value in patients with biochemical recurrence of prostate cancer. Eur J Nucl Med Mol Imaging 2008;35:18-23.
- Rayn KN, Elnabawi YA, Sheth N. Clinical implications of PET/CT in prostate cancer management. Transl Androl Urol. 2018;7(5):844-54.
- Schuste DM, Nieh PT, Jani AB, et al. Anti-3-[18F] FACBC positron emission tomography-computerized tomography and 111In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol. 2014;191(5):!446-53.
- Wondergem M, van der Zant FM, van der Ploeg T, Knol RJJ. A literature review of 18F-fluoride PET/CT and 18F-choline or 11C-choline PET/CT for detection of bone metastases in patients with prostate cancer. Nuclear medicine communications Nucl Med Commun. 2013;34(10):935-45.
- Nanni C, Zanoni L, Pultrone C, et al. 18 F-FACBC (anti1-amino-3-18 F-fluorocyclobutane-1-carboxylic acid) versus 11 C-choline PET/CT in prostate cancer relapse: results of a prospective trial. Eur J Nucl Med Mol Imaging. 2016;43(9):1601-10.
- Afshar-Oromieh A, Malcher A, Eder M, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging 2013;40:486-95.
- Chang SS. Overview of Prostate-Specific Membrane Antigen. Rev Urol. 2004;6(Suppl 10):S13-S18.
- Farolfi A, Calderoni L, Mattana F, et al. Current and Emerging Clinical Applications of PSMA PET Diagnostic Imaging for Prostate Cancer. J Nucl Med. 2021;62:596-604.
- Prostate Cancer. https://uroweb.org/guidelines/prostate-cancer. Accessed on Aug 14, 2022
- Ahmed HU, Bosaily AE, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389(10071):815-822.
- Bodar YJL, Jansen BHE, van der Voorn JP, et al. Detection of prostate cancer with 18 F-DCFPyL PET/CT compared to final histopathology of radical prostatectomy specimens: is PSMA-targeted biopsy feasible? The DeTeCT trial. World J Urol. 2021;39(7):2439-2446.
- Eiber M, Weirich G, Holzapfel K, et al. Simultaneous 68Ga-PSMA HBED-CC PET/MRI Improves the Localization of Primary Prostate Cancer. Eur Urol. 2016;70(5):829-836.
- Emmett L, Buteau J, Papa N, et al. The Additive Diagnostic Value of Prostate-specific Membrane Antigen Positron Emission Tomography Computed Tomography to Multiparametric Magnetic Resonance Imaging Triage in the Diagnosis of Prostate Cancer (PRIMARY): A Prospective Multicentre Study. Eur Urol. 2021;80(6):682-9.
- Lopci E, Saita A, Lazzeri M, et al. 68 Ga-PSMA Positron Emission Tomography/Computerized Tomography for Primary Diagnosis of Prostate Cancer in Men with Contraindications to or Negative Multiparametric Magnetic Resonance Imaging: A Prospective Observational Study. J Urol. 2018;200(1):95-103.
Treatment Intensification in Metastatic Hormone Sensitive Prostate Cancer (mHSPC) Cases - Synchronous High Volume mHSPC
Since 2015, multiple combination treatment strategies have emerged for the management of patients with metastatic hormone sensitive prostate cancer (mHSPC). The addition of docetaxel and/or androgen receptor-axis targeted (ARAT) agents to standard androgen deprivation therapy (ADT), in the form of doublet and triplet treatment strategies, has demonstrated overall survival benefits in this cohort of patients. As such, these drug combinations have changed the standard of care approaches in these men.1
- Written by: Rashid K. Sayyid, MD, MSc and Zachary Klaassen, MD, MSc
- References:
- Weiner AB, Siebert AL, Fenton SE, et al. First-line Systemic Treatment of Recurrent Prostate Cancer After Primary or Salvage Local Therapy: A Systematic Review of the Literature. Eur Urol Oncol. 2022.
- Cancer Stat Facts: Prostate Cancer. National Cancer Institute. Available at https://seer.cancer.gov/statfacts/html/prost.html. Accessed: Nov 14, 2022
- Deek MP, Van der Eecken K, Phillips R, et al. The mutational landscape of metastatic castration-sensitive prostate cancer: the spectrum theory revisited. Eur Urol. 2021;80:632-640
- Stopsack KH, Nandakumar S, Wimber AG, et al. Oncogenic genomic alterations, clinical phenotypes, and outcomes in metastatic castration-sensitive prostate cancer. Clin Cancer Res. 2020;26:3230-3238.
- Sweeney CJ, Chen Y, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N ENgl J Med. 2015;373:737-746.
- Fizai K, Foulon S, Carles J, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet 2022;399(10336):1695-1707.
- Smith MR, Hussain M, Saad F, et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med. 2022;386(12):1132-1142.
- Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med. 2019;381(2):121-131.
- Hoyle AP, Ali A, James ND, et al. Abiraterone in “High-” and “Low-risk” Metastatic Hormone-sensitive Prostate Cancer. Eur Urol. 2018;76(6):719-728.
- James ND, de Bono JS, Spears MR, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med. 2017;377:338-351.
- Chi KN, Chowdhury S, Bjartell A, et al. Apalutamide in Patients With Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J Clin Oncol. 2021;39(2):2294-2303.
- Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol. 2019;37(32):2974-2986.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353-2366.
- Boeve LMS, Hulshof MCCM, Vis AN, et al. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. Eur Urol. 2019;75(3):410-418.
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: A prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36(5):446-453.
- Phillips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020;6(5):650-659.
- Deek MP, van der Eecken K, Sutera P, et al. Long-Term Outcomes and Genetic Predictors of Response to Metastasis-Directed Therapy Versus Observation in Oligometastatic Prostate Cancer: Analysis of STOMP and ORIOLE Trials. J Clin Oncol. 2022;JCO2200644.
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185):2051-2058.
Real-World Utilization of Treatment Intensification in Metastatic Hormone Sensitive Prostate Cancer: Are We Lacking Intensity?
Prostate cancer, while commonly diagnosed early in the disease state, remains the second leading cause of cancer mortality in the United States and Europe1 . Of the 1.3 million new annual diagnoses of prostate cancer, 6% of men have metastases at the time of diagnosis. Such patients are defined as having de novo or synchronous metastatic hormone sensitive prostate cancer (mHSPC).
- Written by: Rashid Sayyid, MD MSc, & Zachary Klaassen, MD MSc
- References:
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502-1512.
- Cancer Stat Facts: Prostate Cancer. National Cancer Institute. Available at https://seer.cancer.gov/statfacts/html/prost.html. Accessed: July 17, 2022.
- James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163-1177.
- Sweeney CJ, Chen Y, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N ENgl J Med. 2015;373:737-746.
- Fizazi K, Tran N, Fein L, et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. New Engl J Med.. 2017;377(4):352-360.
- Hoyle AP, Ali A, James ND, et al. Abiraterone in “High-” and “Low-risk” Metastatic Hormone-sensitive Prostate Cancer. Eur Urol. 2018;76(6):719-728.
- Chi KN, Chowdhury S, Bjartell A, et al. Apalutamide in Patients With Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J Clin Oncol. 2021;39(2):2294-2303.
- Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med. 2019;381(2):121-131.
- Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol. 2019;37(32):2974-2986.
- Fizai K, Foulon S, Carles J, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet 2022;399(10336):1695-1707.
- Smith MR, Hussain M, Saad F, et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med. 2022;386(12):1132-1142.
- Virgo KS, Rumble RB, de Wit R, et al. Initial Management of Noncastrate Advanced, Recurrent, or Metastatic Prostate Cancer: ASCO Guideline Update. J Clin Oncol. 2021;39(11):1274-1305.
- Schaeffer E, Srinivas S, Antonarakis ES, et al. NCCN Guidelines Insights: Prostate Cancer, Version 1.2021. J Natl Compr Canc Netw. 2021;19(2):134-143.
- EAU Guidelines Prostate Cancer. https://uroweb.org/guidelines/prostate-cancer. Accessed on Oct 1, 2022.
- Ryan CJ, Ke X, Lafeuille M, et al. Management of Patients with Metastatic Castration-Sensitive Prostate Cancer in the Real-World Setting in the United States/ J Urol. 2021;206(6):1420-1429.
- Freedland SJ, Sandin R, Sah J, et al. Treatment patterns and survival in metastatic castration-sensitive prostate cancer in the US Veterans Health Administration. Cancer Med. 2021;10(23):8570-8580.
- Leith A, Ribbands A, Kim J, et al. Impact of next-generation hormonal agents on treatment patterns among patients with metastatic hormone-sensitive prostate cancer: a real-world study from the United States, five European countries and Japan. BMC Urol. 2022;22(1):33.
- Wallis CJD, Malone S, Cagiannos I, et al. Real-World Use of Androgen-Deprivation Therapy: Intensification Among Older Canadian Men With de Novo Metastatic Prostate Cancer. JNCI Cancer Spectr. 2021;5(6):pkab082.
The Importance of a Prostate Cancer Survivorship Program: A Multi-Disciplinary Approach
- Written by: Zachary Klaassen, MD, MSc & Sherita A. King, MD
- References:
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34.
- Hamdy FC, Donovan JL, Lane JA, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med. 2016;375(15):1415-1424.
- Donovan JL, Hamdy FC, Lane JA, et al. Patient-Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N Engl J Med. 2016;375(15):1425-1437.
- Albkri A, Girier D, Mestre A, Costa P, Droupy S, Chevrot A. Urinary Incontinence, Patient Satisfaction, and Decisional Regret after Prostate Cancer Treatment: A French National Study. Urol Int. 2018;100(1):50-56.
- Pinkawa M, Berneking V, Schlenter M, Krenkel B, Eble MJ. Quality of Life After Radiation Therapy for Prostate Cancer With a Hydrogel Spacer: 5-Year Results. Int J Radiat Oncol Biol Phys. 2017;99(2):374-377.
- Wallis CJ, Mahar AL, Satkunasivam R, et al. Cardiovascular and Skeletal-related Events Following Localized Prostate Cancer Treatment: Role of Surgery, Radiotherapy, and Androgen Deprivation. Urology. 2016;97:145-152.
- Ravi P, Karakiewicz PI, Roghmann F, et al. Mental health outcomes in elderly men with prostate cancer. Urol Oncol. 2014;32(8):1333-1340.
- Watts S, Leydon G, Birch B, et al. Depression and anxiety in prostate cancer: a systematic review and meta-analysis of prevalence rates. BMJ Open. 2014;4(3):e003901.
- Marzouk K, Assel M, Ehdaie B, Vickers A. Long-Term Cancer Specific Anxiety in Men Undergoing Active Surveillance of Prostate Cancer: Findings from a Large Prospective Cohort. J Urol. 2018;200(6):1250-1255.
- Dinh KT, Reznor G, Muralidhar V, et al. Association of Androgen Deprivation Therapy With Depression in Localized Prostate Cancer. J Clin Oncol. 2016;34(16):1905-1912.
- Nead KT, Sinha S, Yang DD, Nguyen PL. Association of androgen deprivation therapy and depression in the treatment of prostate cancer: A systematic review and meta-analysis. Urol Oncol. 2017;35(11):664 e661-664 e669.
- Klaassen Z, Jen RP, DiBianco JM, et al. Factors associated with suicide in patients with genitourinary malignancies. Cancer. 2015;121(11):1864-1872.
- 13.Dalela D, Krishna N, Okwara J, et al. Suicide and accidental deaths among patients with non-metastatic prostate cancer. BJU Int. 2016;118(2):286-297.
- Guo Z, Gan S, Li Y, et al. Incidence and risk factors of suicide after a prostate cancer diagnosis: a meta-analysis of observational studies. Prostate Cancer Prostatic Dis. 2018;21(4):499-508.
- Klaassen Z, Wallis CJ, Goldberg H, et al. Utilization of Psychiatric Resources Prior to Genitourinary (GU) Cancer Diagnosis: Implications for Survival Outcomes. AUA 2019. 2019.
- Fashoyin-Aje LA, Martinez KA, Dy SM. New patient-centered care standards from the commission on cancer: opportunities and challenges. J Support Oncol. 2012;10(3):107-111.
- Skolarus TA, Wolf AM, Erb NL, et al. American Cancer Society prostate cancer survivorship care guidelines. CA Cancer J Clin. 2014;64(4):225-249.
- Resnick MJ, Lacchetti C, Bergman J, et al. Prostate cancer survivorship care guideline: American Society of Clinical Oncology Clinical Practice Guideline endorsement. J Clin Oncol. 2015;33(9):1078-1085.
- Sharpley CF, Bitsika V, Christie DR, Hunter MS. Measuring depression in prostate cancer patients: does the scale used make a difference? Eur J Cancer Care (Engl). 2017;26(1).
- National Comprehensive Cancer N. Distress management. Clinical practice guidelines. J Natl Compr Canc Netw. 2003;1(3):344-374.
- Klaassen Z, Arora K, Wilson SN, et al. Decreasing suicide risk among patients with prostate cancer: Implications for depression, erectile dysfunction, and suicidal ideation screening. Urol Oncol. 2018;36(2):60-66.
- Recklitis CJ, Zhou ES, Zwemer EK, Hu JC, Kantoff PW. Suicidal ideation in prostate cancer survivors: understanding the role of physical and psychological health outcomes. Cancer. 2014;120(21):3393-3400.
- Wittmann D, Varlamos C, Rodriguez-Galano N, et al. Developing a Patient-Centered Model of Prostate Cancer Care: Patient Satisfaction With a Survivorship Program Embedded in Urologic-Oncologic Care. Urology. 2022;160:161-167.
The Current Landscape of Metastatic Castration-Resistant Prostate Cancer: Radioligand Therapy
- Written by: Rashid Sayyid, MD MSc, & Zachary Klaassen, MD MSc
- References:
- Sartor O. Isotope Therapy for Castrate-Resistant Prostate Cancer: Unique Sequencing and Combinations. Cancer J 2016; 22(5):342-346.
- Ye X, Sun D, Lou C. Comparison of the efficacy of strontium-89 chloride in treating bone metastasis of lung, breast, and prostate cancers. J Cancer Res Ther 2018; 14(Supplement):S36-S40.
- James N, Pirrie S, Pope A, et al. TRAPEZE: a randomised controlled trial of the clinical effectiveness and cost-effectiveness of chemotherapy with zoledronic acid, strontium-89, or both, in men with bony metastatic castration-refractory prostate cancer. Health Technol Assess 2016; 20(53):1-288.
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-223.
- Chang SS. Overview of Prostate-Specific Membrane Antigen. Rev Urol. 2004;6(Suppl 10):S13-S18.
- Hofman MS, Violet J, Hicks RJ, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825-833.
- Seifert R, Kessel K, Schlack K, Weckesser M, Bogemann M, Rahbar K. Radioligand therapy using [(177)Lu]Lu-PSMA-617 in mCRPC: a pre-VISION single-center analysis. Eur J Nucl Med Mol Imaging. 2020;47(9):2106-2112.
- Hofman MS, Emmett L, Sandhu S, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397(10276):797-804.
- Gaertner FC, Halabi K, Ahmadzadehfar H, et al. Uptake of PSMA-ligands in normal tissues is dependent on tumor load in patients with prostate cancer. Oncotarget. 2017;8(33):55094-55103.
- Sartor O, de Bono J, Chi KN, et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021;385:1901-1103.
- Hartrampf PE, Seitz AK, Weinzierl F, et al. Baseline clinical characteristics predict overall survival in patients undergoing radioligand therapy with [ 177 Lu]Lu-PSMA I&T during long-term follow-up. Eur J Ncul Med Mol Imaging. 2022.
Radiotherapy in Prostate Cancer: Concurrent Use of Systemic Therapy with Radiotherapy in Localized and Locally Advanced Disease
External beam radiotherapy, along with radical prostatectomy, has been a mainstay treatment option for prostate cancer for decades and is currently recommended by numerous guidelines for the treatment of intermediate- and high-risk disease.1-3 Unlike surgery which is often, at least initially, planned as a monotherapy, many patients who opt for external beam radiotherapy will receive concurrent therapy. This center of excellence article will review the evidence for concurrent systemic therapy with radiotherapy in men with localized and locally advanced disease.
- Written by: Rashid Sayyid, MD MSc, & Zachary Klaassen, MD MSc
- References:
- Eastham JA, Auffenberg GB, Barocas DA, et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part I: Introduction, Risk Assessment, Staging, and Risk-Based Management. J Urol. 2022;208(1):10-18.
- Schaeffer E, Srinivas S, Antonarakis ES, et al. NCCN Guidelines Insights: Prostate Cancer, Version 1.2021. J Natl Compr Canc Netw. 2021;19(2):134-143.
- EAU: Prostate Cancer. https://uroweb.org/guidelines/prostate-cancer. Accessed on Oct 8, 2022.
- Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;60(9327):103-106
- Warde P, Mason M, Ding K, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet. 2011;378(9809);2104-2111.
- D'Amico AV, Chen MH, Renshaw A, Loffredo M, Kantoff PW. Long-term Follow-up of a Randomized Trial of Radiation With or Without Androgen Deprivation Therapy for Localized Prostate Cancer. JAMA. 2015;314(12):1291-1293.
- D’amico AV, Chen M, Renshaw, et al. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008;299(3):289-295.
- Bolla M, Maingon P, Carrie C, et al. Short Androgen Suppression and Radiation Dose Escalation for Intermediate- and High-Risk Localized Prostate Cancer: Results of EORTC Trial 22991. J Clin Oncol. 2016;34(15):1748-1756.
- Zapatero A, Guerrero A, Maldonado X, et al. High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16(3):320-327.
- Denham JW, Joseph D, Lamb DS, et al. Short-term androgen suppression and radiotherapy versus intermediate-term androgen suppression and radiotherapy, with or without zoledronic acid, in men with locally advanced prostate cancer (TROG 03.04 RADAR): 10-year results from a randomised, phase 3, factorial trial. Lancet Oncol. 2019;20(2):267-281.
- Nabid A, Carrier N, Martin AG, et al. Duration of Androgen Deprivation Therapy in High-risk Prostate Cancer: A Randomized Phase III Trial. Eur Urol. 2018;74(4):432-441.
- Kishan AU, Sun Y, Hartman H, et al. Androgen deprivation therapy use and duration with definitive radiotherapy for localised prostate cancer: an individual patient data meta-analysis. Lancet Oncol. 2022;23(2):304-316.
- Kishan AU, Wang X, Sun Y, et al. High-dose Radiotherapy or Androgen Deprivation Therapy (HEAT) as Treatment Intensification for Localized Prostate Cancer: An Individual Patient–data Network Meta-analysis from the MARCAP Consortium. Eur Urol. 2022;82(1):106-114.
- James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163-77.
- Sweeney CJ, Chen Y, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med. 2015;373:737-746.
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502-1512.
- Morris WJ, Pickles T, Keyes M. Using a surgical prostate-specific antigen threshold of >0.2 ng/mL to define biochemical failure for intermediate- and high-risk prostate cancer patients treated with definitive radiation therapy in the ASCENDE-RT randomized control trial. Brachytherapy. 2018;17(6):837-844.
- Kellokumpu-Lehtinen PL, Hjälm-Eriksson M, Thellenberg-Karlsson C, et al. Investigators of the Scandinavian Prostate Cancer Study No. 13. Docetaxel versus surveillance after radical radiotherapy for intermediate- or high-risk prostate cancer-results from the prospective, randomised, open-label phase III SPCG-13 trial. Eur Urol. 2019;76:823-830.
- Fizazi K, Carmel A, Joly F, et al. Updated results of GETUG-12, a phase III trial of docetaxel-based chemotherapy in high-risk localized prostate cancer, with a 12-year follow-up. Ann Oncol. 2018;29:viii271-viii302.
- Rosenthal SA, Hu C, Sartor O, et al. Effect of Chemotherapy With Docetaxel With Androgen Suppression and Radiotherapy for Localized High-Risk Prostate Cancer: The Randomized Phase III NRG Oncology RTOG 0521 Trial. J Clin Oncol. 2019;37(14):1159-1168.
- D’Amico AV, Xie W, McMahon E, et al. Radiation and Androgen Deprivation Therapy With or Without Docetaxel in the Management of Nonmetastatic Unfavorable-Risk Prostate Cancer: A Prospective Randomized Trial. J Clin Oncol. 2021;39(26):2938-2947.
- McBride SM, Spratt DE, Kollmeier M, et al. Interim results of aasur: A single arm, multi-center phase 2 trial of apalutamide (A) + abiraterone acetate + prednisone (AA+P) + leuprolide with stereotactic ultra-hypofractionated radiation (UHRT) in very high risk (VHR), node negative (N0) prostate cancer (PCa). J Clin Oncol. 2021 ASCO Annual Meeting.
- Attard G, Murphy L, Clarke NW, et al. Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: a meta-analysis of primary results from two randomised controlled phase 3 trials of the STAMPEDE platform protocol. Lancet. 2022;399(10323):447-460.
- Nguyen PL, Huang HC, Davicioni E, et al. Validation of a 22-gene Genomic Classifier in the NRG Oncology/RTOG 9202, 9413 and 9902 Phase III Randomized Trials: A Biopsy-Based Individual Patient Meta-Analysis in High-Risk Prostate Cancer. Int J Rad Oncol Biol Phys. 2021;111(3):PS50.
The Current Landscape of Metastatic Castration-Resistant Prostate Cancer: Immunotherapy and Targeted Therapies
- Written by: Rashid Sayyid, MD, MSc, & Zachary Klaassen, MD, MSc
- References:
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411-422.
- FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication. Accessed on Aug 6, 2022.
- Abida W, Cheng ML, Armenia J, et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019;5(4):471-478.
- Pritchard CC, Morrissey C, Kumar A,et al. Complex MSH2and MSH6mutations in hypermutated microsatellite unstable advanced prostate cancer. Nat Commun. 2014;5:4988.
- National Comprehensive Cancer Network . Prostate Cancer (Version 4.2022). https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed Aug 6, 2022.
- Antonarakis ES, Piulats JM, Gross-Goupil M, et al. Pembrolizumab for Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study. J Clin Oncol. 2020;38(5):395-405.
- Rubin MA, Maher CA, Chinnaiyan AM. Common gene rearrangements in prostate cancer. J Clin Oncol. 2011;29(27):3659-3668.
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N Engl J Med. 20116;375:443-453.
- Kunkel TA, Erie DA. DNA mismatch repair. Annu Rev Biochem. 2005;74:681-710.
- Abida W, Patnaik A, Campbell D, et al. Rucaparib in Men With Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J Clin Oncol. 2020;38(32):3763-3772.
- Abida W, Campbell D, Patnaik A, et al. Non-BRCA DNA Damage Repair Gene Alterations and Response to the PARP Inhibitor Rucaparib in Metastatic Castration-Resistant Prostate Cancer: Analysis From the Phase II TRITON2 Study. Clin Cancer Res. 2020;26(11):2487-96.
- Hussain M, Mateo J, Fizazi K, et al. Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;383:2345-57.
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;382:2091-2102.
- Thiery-Vuillemin A, de Bono J, Hussain M, et al. Pain and health-related quality of life with olaparib versus physician's choice of next-generation hormonal drug in patients with metastatic castration-resistant prostate cancer with homologous recombination repair gene alterations (PROfound): an open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23(3):393-405.
- de Bono JS, Mehra N, Scagliotti GV, et al. Talazoparib monotherapy in metastatic castration-resistant prostate cancer with DNA repair alterations (TALAPRO-1): an open-label, phase 2 trial Lancet Oncol. 2021;22(9):1250-1264.
- Gasmi A, Roubaud G, Dariane C, et al. Overview of the Development and Use of Akt Inhibitors in Prostate Cancer. J Clin Med. 2022;11(1):160.
- De Bono JS, De Giorgi U, Rodrigues DN, et al. Randomized Phase II Study Evaluating Akt Blockade with Ipatasertib, in Combination with Abiraterone, in Patients with Metastatic Prostate Cancer with and without PTEN Loss. Clin Cancer Res. 2019;25:928–936.
- Sweeney C, Bracarda S Sternberg CN, et al. Ipatasertib plus abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2021;398(10295):131-142.
- Crabb SJ, Griffiths G, Marwood E, et al. Pan-AKT Inhibitor Capivasertib With Docetaxel and Prednisolone in Metastatic Castration-Resistant Prostate Cancer: A Randomized, Placebo-Controlled
Radiotherapy in Prostate Cancer: Utilization in the Metastatic Setting
While external beam radiotherapy is a standard treatment option as first-line therapy for men with localized prostate cancer, it has been more recently recognized as an important component in the care of men with metastatic prostate cancer. This Center of Excellence article will explore recent evidence for the utilization of radiotherapy in the metastatic setting.
- Written by: Rashid Sayyid, MD MSc & Zachary Klaassen, MD MSc
- References:
- McAllister SS, Gifford AM, Greiner AL, et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008;133:994-1005.
- Boeve LMS, Hulshof MCCM, Vis AN, et al. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. Eur Urol. 2019;75(3):410-418.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353-2366.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the prostate for men with metastatic prostate cancer in the UK and Switzerland: Long-term results from the STAMPEDE randomised controlled trial. PLoS Medicine. 2022;19(6):e1003998.
- Ali A, Hoyle A, Haran AM, et al. Association of Bone Metastatic Burden With Survival Benefit From Prostate Radiotherapy in Patients With Newly Diagnosed Metastatic Prostate Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2021;7(4):555-563.
- Fizazi K, Tran N, Fein L, et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. New Engl J Med.. 2017;377(4):352-360.
- Hoyle AP, Ali A, James ND, et al. Abiraterone in “High-” and “Low-risk” Metastatic Hormone-sensitive Prostate Cancer. Eur Urol. 2018;76(6):719-728.
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: A prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36(5):446-453.
- Phillips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020;6(5):650-659.
- Deek MP, van der Eecken K, Sutera P, et al. Long-Term Outcomes and Genetic Predictors of Response to Metastasis-Directed Therapy Versus Observation in Oligometastatic Prostate Cancer: Analysis of STOMP and ORIOLE Trials. J Clin Oncol. 2022;JCO2200644.
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185):2051-2058.
- Palma DA, Olson R, Harrow S, et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J Clin Oncol. 2020;38(25):2830-2838.
- Supiot S, Vaugier L, Pasquier D, et al. OLIGOPELVIS GETUG P07, a Multicenter Phase II Trial of Combined High-dose Salvage Radiotherapy and Hormone Therapy in Oligorecurrent Pelvic Node Relapses in Prostate Cancer. Eur Urol. 2021;80(4):405-414.
The Current Landscape of Metastatic Castration-Resistant Prostate Cancer: Novel Hormonal Therapies
While the emergence of castration resistant disease comes as a result of the disease progressing in spite of castrate levels of testosterone (at times called hormone refractory disease), prostate cancer (even in the castration resistance prostate cancer (CRPC) setting) remains heavily dependent on the androgen axis.
- Written by: Rashid Sayyid, MD MSc, & Zachary Klaassen, MD MSc
- References:
- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995-2005
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138-148.
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. New Engl J Med. 2012;367(13):1187-1197.
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424-433.
- Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in Men with Chemotherapy-naive Metastatic Castration-resistant Prostate Cancer: Extended Analysis of the Phase 3 PREVAIL Study. Eur Urol. 2016.
- Denmeade SR, Wang H, Agarwal N, et al. TRANSFORMER: A Randomized Phase II Study Comparing Bipolar Androgen Therapy Versus Enzalutamide in Asymptomatic Men With Castration-Resistant Metastatic Prostate Cancer. J Clin Oncol. 2021;39(12):1371-1382.
- Bipolar Androgen Therapy in Men with Metastatic Castration-resistant Prostate Cancer (RESTORE): A Comparison of Post-abiraterone Versus Post-enzalutamide Cohorts. Eur Urol. 2021;692-699.
- Khalaf DJ, Annala M, Taavitsainen S, et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol. 2019l20(12):1730-1739.
- Saad F, Efstathiou E, Attard G, et al. Apalutamide plus abiraterone acetate and prednisone versus placebo plus abiraterone and prednisone in metastatic, castration-resistant prostate cancer (ACIS): a randomised, placebo-controlled, double-blind, multinational, phase 3 study. Lancet Oncol. 2021;22(11):1541-1559.
- Colomba E, Jonas SF, Eymard J-C, et al. Objective computerized cognitive assessment in men with metastatic castrate-resistant prostate cancer (mCRPC) randomly receiving darolutamide or enzalutamide in the ODENZA trial. Ann Oncol. 2021;32(5):S66-647.
- Cathomas R, Procopio G, Hayoz S, et al. Darolutamide maintenance in metastatic castration resistant prostate cancer (mCRPC) previously treated with novel hormonal agents (NHA) and non-progressive disease after subsequent treatment with a taxane: A randomized double-blind placebo-controlled phase II trial (SAKK 08/16). Ann Oncol. 2021;32(5):S1301-1302.
Radiotherapy in Prostate Cancer: The Role of Pelvic Nodal Irradiation and Focal Boost to the Intraprostatic Tumor
External beam radiotherapy, along with radical prostatectomy, has been a mainstay treatment option for prostate cancer for decades and is currently recommended by numerous guidelines for the treatment of intermediate- and high-risk disease.1-3 While it is clear that radiotherapy should include the tumor, the prostate, and seminal vesicles, the role of prophylactic pelvic nodal irradiation for patients without overt evidence of regional pelvic nodal involvement has long been debated.
- Written by: Rashid Sayyid, MD MSc, & Zachary Klaassen, MD MSc
- References:
- Eastham JA, Auffenberg GB, Barocas DA, et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part I: Introduction, Risk Assessment, Staging, and Risk-Based Management. J Urol. 2022;208(1):10-18.
- Schaeffer E, Srinivas S, Antonarakis ES, et al. NCCN Guidelines Insights: Prostate Cancer, Version 1.2021. J Natl Compr Canc Netw. 2021;19(2):134-143.
- EAU: Prostate Cancer. https://uroweb.org/guidelines/prostate-cancer. Accessed on Oct 8, 2022.
- Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395(10231):1208-1216.
- Roach M III, Marquex C, Yuo HS, et al. Predicting the risk of lymph node involvement using the pre-treatment prostate specific antigen and Gleason score in men with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 1994;28:33-37.
- Roach M, Moughan J, Lawton CA, et al. Sequence of hormonal therapy and radiotherapy field size in unfavourable, localised prostate cancer (NRG/RTOG 9413): long-term results of a randomised, phase 3 trial. Lancet Oncol. 2018;19(11):1504-1515.
- Pommier P, Chabaud S, Lagrange J, et al. Is There a Role for Pelvic Irradiation in Localized Prostate Adenocarcinoma? Update of the Long-Term Survival Results of the GETUG-01 Randomized Study. Int J Radiat Oncol Biol Phys. 2016;96(4):759-769.
- Murthy V, Maitre P, Kannan S, et al. Prostate-Only Versus Whole-Pelvic Radiation Therapy in High-Risk and Very High-Risk Prostate Cancer (POP-RT): Outcomes From Phase III Randomized Controlled Trial. J Clin Oncol. 2021;39(11):1234-42.
- Glicksman RM, Loblaw A, Morton G, et al. Elective pelvic nodal irradiation with a simultaneous hypofractionated integrated prostate boost for localized high risk prostate cancer: Long term results from a prospective clinical trial. Radiotherap Oncol. 2021;163:21-31.
- Glicksman RM, Liu SK, Cheung P, et al. Elective nodal ultra hypofractionated radiation for prostate cancer: Safety and efficacy from four prospective clinical trials. Radiother Oncol. 2021;163:159-164.
- Wallis CJD, Huang LC, Zhao Z, et al. Association between pelvic nodal radiotherapy and patient-reported functional outcomes through 5 years among men undergoing external-beam radiotherapy for prostate cancer: An assessment of the comparative effectiveness analysis of surgery and radiation (CEASAR) cohort. Urol Oncol. 2022;40(2):56.e1-56.e8.
- Kerkmeijer LGW, Groen VH, Pos FJ, et al. Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients With Localized Prostate Cancer: Results From the FLAME Randomized Phase III Trial. J Clin Oncol. 2021;39(7):787-796.
- Wang S, Tang W, Luo H, et al. Efficacy and Toxicity of Whole Pelvic Radiotherapy Versus Prostate-Only Radiotherapy in Localized Prostate Cancer: A Systematic Review and Meta-Analysis. Front Oncol. 2022;11:796907.
The Current Landscape of Metastatic Castration-Resistant Prostate Cancer: Nearly Two Decades of Treatment Options
Prostate cancer, while commonly diagnosed as localized disease, remains the second leading cause of cancer mortality in the United States and Europe.1 For patients who die of prostate cancer, some will be initially diagnosed and treated for metastatic hormone-sensitive disease (mHSPC).
- Written by: Zachary Klaassen, MD MSc and Rashid Sayyid, MD MSc
- References:
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502-1512.
- Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996:14(6):1756-1764.
- Osoba D, Tannock IF, Ernst DS, Neville AJ. Health-related quality of life in men with metastatic prostate cancer treated with prednisone alone or mitoxantrone and prednisone. J Clin Oncol. 1999;17(6):1654-1663.
- de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147-1154.
- de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N Engl J Med. 2019;381(26):2506-2518.
- Morgans AK, Hutson T, Guan AKD, et al. An economic evaluation of cabazitaxel versus a second androgen receptor-targeted agent (ARTA) for patients with metastatic castration-resistant prostate cancer previously treated with docetaxel and an ARTA: the United States payer perspective. BMC Health Serv Res. 2022;22(1):916.
Radiotherapy in Prostate Cancer: Hypofractionation for Clinically Localized Disease
External beam radiotherapy, along with radical prostatectomy, has been a mainstay treatment option for prostate cancer and is currently recommended by numerous guidelines for the treatment of intermediate- and high-risk disease.1-3 While external beam radiotherapy has been foundational in prostate cancer treatment for decades, there have been significant changes in the delivery of radiotherapy, corresponding to technical advances.
- Written by: Rashid Sayyid, MD MSc, & Zachary Klaassen, MD MSc
- References:
- Eastham JA, Auffenberg GB, Barocas DA, et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part I: Introduction, Risk Assessment, Staging, and Risk-Based Management. J Urol. 2022;208(1):10-18.
- Schaeffer E, Srinivas S, Antonarakis ES, et al. NCCN Guidelines Insights: Prostate Cancer, Version 1.2021. J Natl Compr Canc Netw. 2021;19(2):134-143.
- EAU: Prostate Cancer. https://uroweb.org/guidelines/prostate-cancer. Accessed on Oct 8, 2022.
- Wolf F, Sedlmayer F, Abersold D, et al. Ultrahypofractionation of localized prostate cancer : Statement from the DEGRO working group prostate cancer. Strahlenther Onkol. 2021;197(2):89-96.
- Bentzen SM, Lundbeck F, Christensen LL, Overgaard J. Fractionation sensitivity and latency of late radiation injury to the mouse urinary bladder. Radiother Oncol. 1992;25(4):301–307
- Dorr W, Bentzen SM. Late functional response of mouse urinary bladder to fractionated X‑irradiation. Int J Radiat Biol. 1999;75(10):1307–1315
- Marzi S, Saracino B, Petrongari MG, et al. Modeling of alpha/beta for late rectal toxicity from a randomized phase II study: conventional versus hypofractionated scheme for localized prostate cancer. J Exp Clin Cancer Res. 2009;28:117.
- Tucker SL, Thames HD, Michalski JM, et al. Estimation of alpha/beta for late rectal toxicity based on RTOG 94–06. Int J Radiat Oncol Biol Phys. 2011;81(2):600–605.
- Lee WR, Dignam JJ, Amin MB, et al. Randomized Phase III Noninferiority Study Comparing Two Radiotherapy Fractionation Schedules in Patients With Low-Risk Prostate Cancer. J Clin Oncol. 2016;34(20):2325-2332.
- Inrocci L, Wortel RC, Alemayehu WG, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17(8):1061-1069.
- Catton CN, Lukka H, Gu C, et al. Randomized Trial of a Hypofractionated Radiation Regimen for the Treatment of Localized Prostate Cancer. J Clin Oncol. 2017;35(17):1884-1890.
- Wang MH, Vos LJ, Yee D, et al. Clinical Outcomes of the CHIRP Trial: A Phase II Prospective Randomized Trial of Conventionally Fractionated Versus Moderately Hypofractionated Prostate and Pelvic Nodal Radiation Therapy in Patients With High-Risk Prostate Cancer. Pract Radiat Oncol. 2021;11(5):384-393.
- Dearnaley D, Syndikus I, Mosspo H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047-1060.
- Widmark A, Gunnlaugsson A, Beckman L, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet. 2019;394(10196):385-395.
- Kishan AU, Wang X, Sun Y, et al. High-dose Radiotherapy or Androgen Deprivation Therapy (HEAT) as Treatment Intensification for Localized Prostate Cancer: An Individual Patient–data Network Meta-analysis from the MARCAP Consortium. Eur Urol. 2022;82(1):106-114.
- Brand DH, Tree AC, Ostler P, et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. 2019;20(11):1531-1543.
Metastatic Hormone-Sensitive Prostate Cancer: Impact of Disease Volume and Timing of Metastases
Introduction
Since 2015, the metastatic hormone sensitive prostate cancer (mHSPC) disease space now has several options of doublet and triplet therapy, using ADT as the backbone of treatment, leading to an overall survival (OS) advantage versus ADT alone. Thus, this has changed the standard of care for treatment intensification for these men. The incidence of metastatic prostate cancer at diagnosis ranges from ~5-50%, with vast geographic differences. Such patients are defined as having de novo or synchronous mHSPC. Additionally, some men who are initially diagnosed with non-metastatic disease will have progression to metastasis prior to development of castration resistance, also known as metachronous mHSPC.1This distinction between synchronous and metachronous mHSPC is of utmost clinical importance given differences in prognosis,2 genomic mutational profiles,3,4 and recommended systemic treatment options between these two groups.3 Both groups can be further stratified by volume of metastatic disease, most commonly by using the CHAARTED high-volume criteria as follows: presence of visceral metastases or ≥4 bone lesions with ≥1 beyond the vertebral bodies and pelvis.5 As such four distinct subgroups become clinically relevant (median OS per CHAARTED and GETUG-15 among men receiving ADT alone, ie. the control groups in these trials):
- Metachronous and low volume: ~8 years
- Metachronous and high volume: 4.5 years
- Synchronous and low volume: 4.5 years
- Synchronous and high volume: 3 years
Given that the results of the key phase 3 mHSPC randomized clinical trials in the intention to treat population are discussed in another Center of Excellence article, the following article will take on more a nuanced approach assessing outcomes of these trials focusing specifically on timing of metastatic disease (de novo versus metachronous) and volume of disease (high versus low). Additionally, we will address several specific treatment considerations (ie. metastasis directed therapy [MDT] and prostate radiotherapy) for those with very low, or oligometastatic, mHSPC.
ADT + Docetaxel
The CHAARTED trial was initially published in 2015 and an updated analysis was published in 2018 evaluating the survival benefit of docetaxel addition to ADT by volume status.6 This trial was enriched for high volume disease (64.9% of total cohort) and 72.8% of patients presented with de novo disease. This updated analysis demonstrated that the benefit of docetaxel addition is restricted to patients with high volume disease (median OS 51.2 months for docetaxel + ADT versus 34.4 months for ADT alone; HR 0.63, 95% CI 0.50 to 0.79) but not low volume disease (median OS 63.5 months for docetaxel + ADT versus not reached for ADT alone; HR: 1.04, 95% CI 0.70 to 1.55):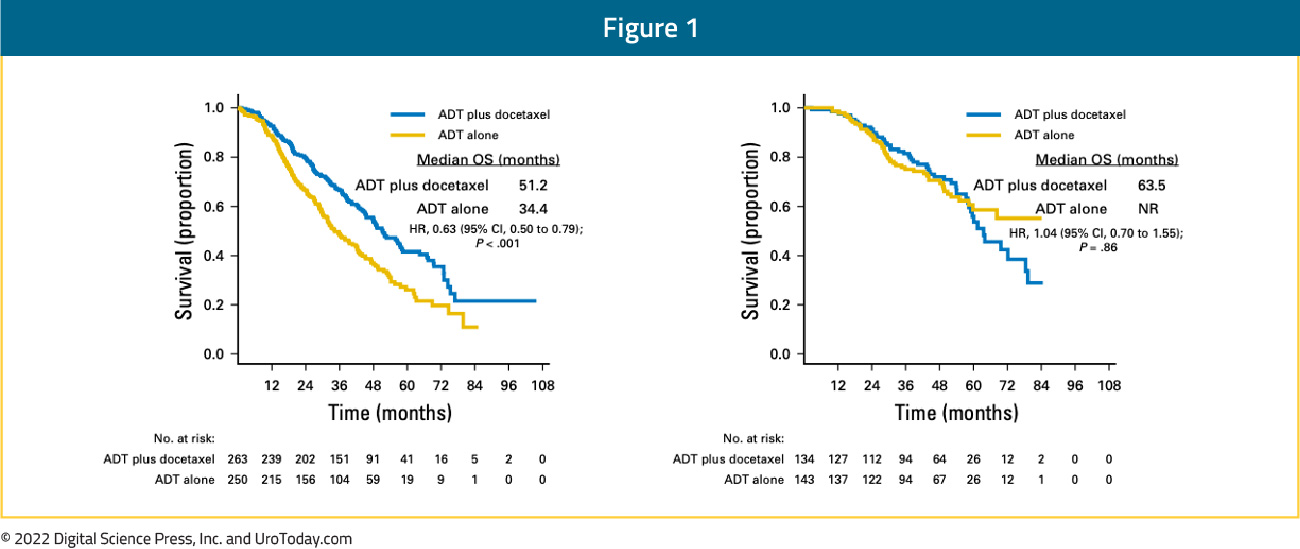
Patients with high volume disease appeared to derive a benefit from docetaxel addition irrespective of whether they present with de novo metastatic disease (median OS 48.0 versus 33.1 months; HR 0.63, 95% CI 0.59 to 0.81) or metastatic recurrence following prior local therapy (median OS 66.9 versus 51.7 months; HR 0.72, 95% CI 0.36 to 1.46). There was no benefit seen in the low volume groups, irrespective of whether presenting with de novo or recurrent mHSPC.6 The most recent update at ASCO 2022 presented the 8-year OS data for patients from the CHAARTED trial. This update confirmed an OS benefit for docetaxel addition in the high-volume cohort, however, it also demonstrated a mortality benefit for synchronous low-volume mHSPC patients with OS rates of 44.6% versus 40.9% (HR 0.77, 95% CI 0.51 to 1.18), but not metachronous low-volume patients (43.4% versus 64.2%; HR 1.65, 95% CI 0.95 to 2.87):
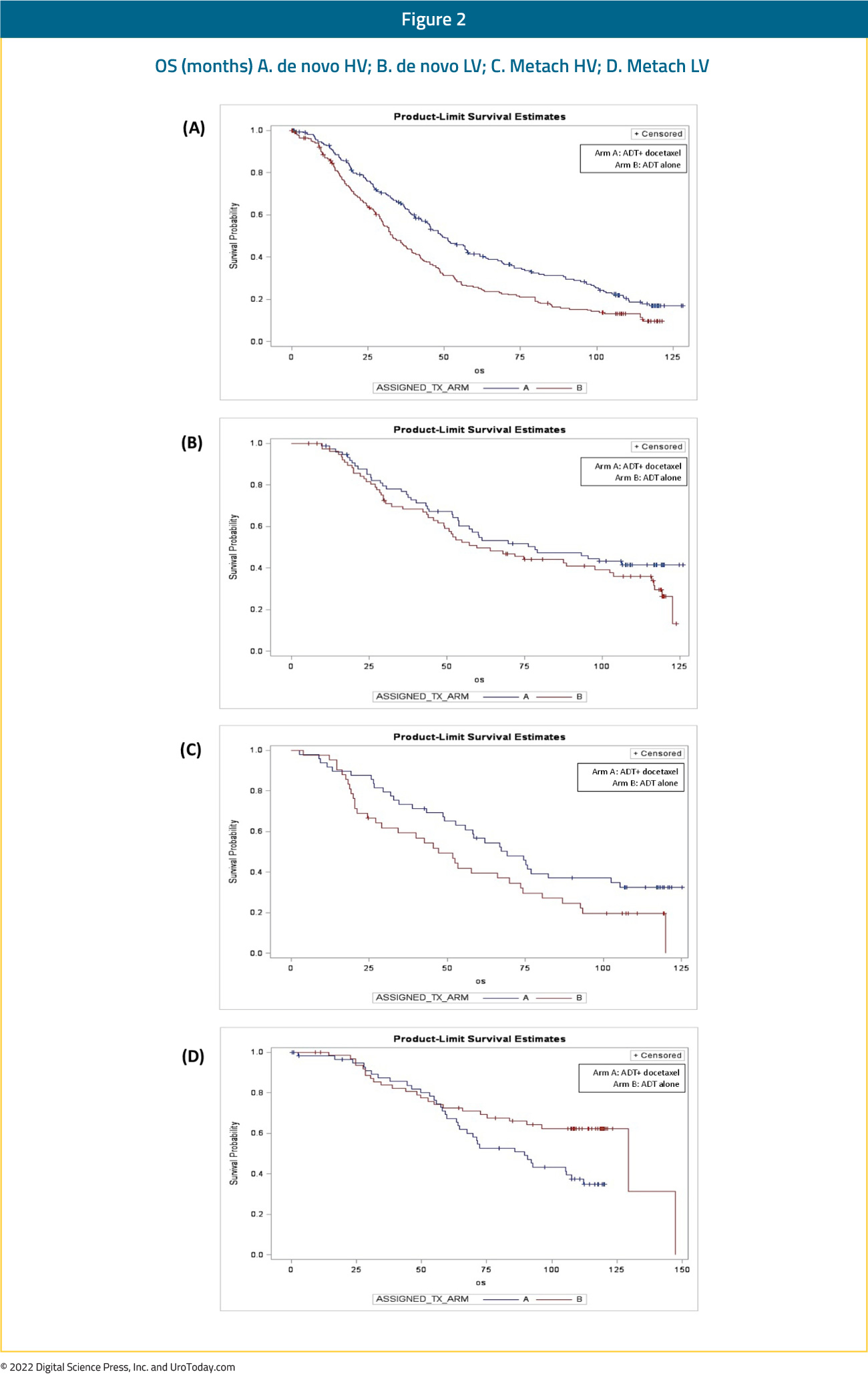
The GETUG-15 trial included 70.6% patients with de novo mHSPC, with only 50% of patients considered high volume per the CHAARTED criteria (compared to 64.9% in CHAARTED). This may in part explain the negative OS outcome seen in GETUG-15. Long-term follow-up from GETUG7 and combined analysis from GETUG-15 and CHAARTED8 demonstrated benefit for docetaxel addition in the high volume mHSPC, but not those with low-volume disease.
Updated results from the STAMPEDE trial published in 2019 with a median follow up of 78.2 months retrospectively evaluated imaging results for patients with available baseline staging scans (76%). This demonstrated consistent OS benefits for docetaxel in both the low and high-volume cohorts (CHAARTED criteria). In low volume patients, median OS improved from 76.7 months to 93.2 months with docetaxel (HR 0.76, 95% CI 0.54 to 1.07) compared to 39.9 months from 35.2 months in the high-volume patients (HR 0.81, 95% CI 0.64 to 1.02) (interaction by metastatic burden p=0.827).9
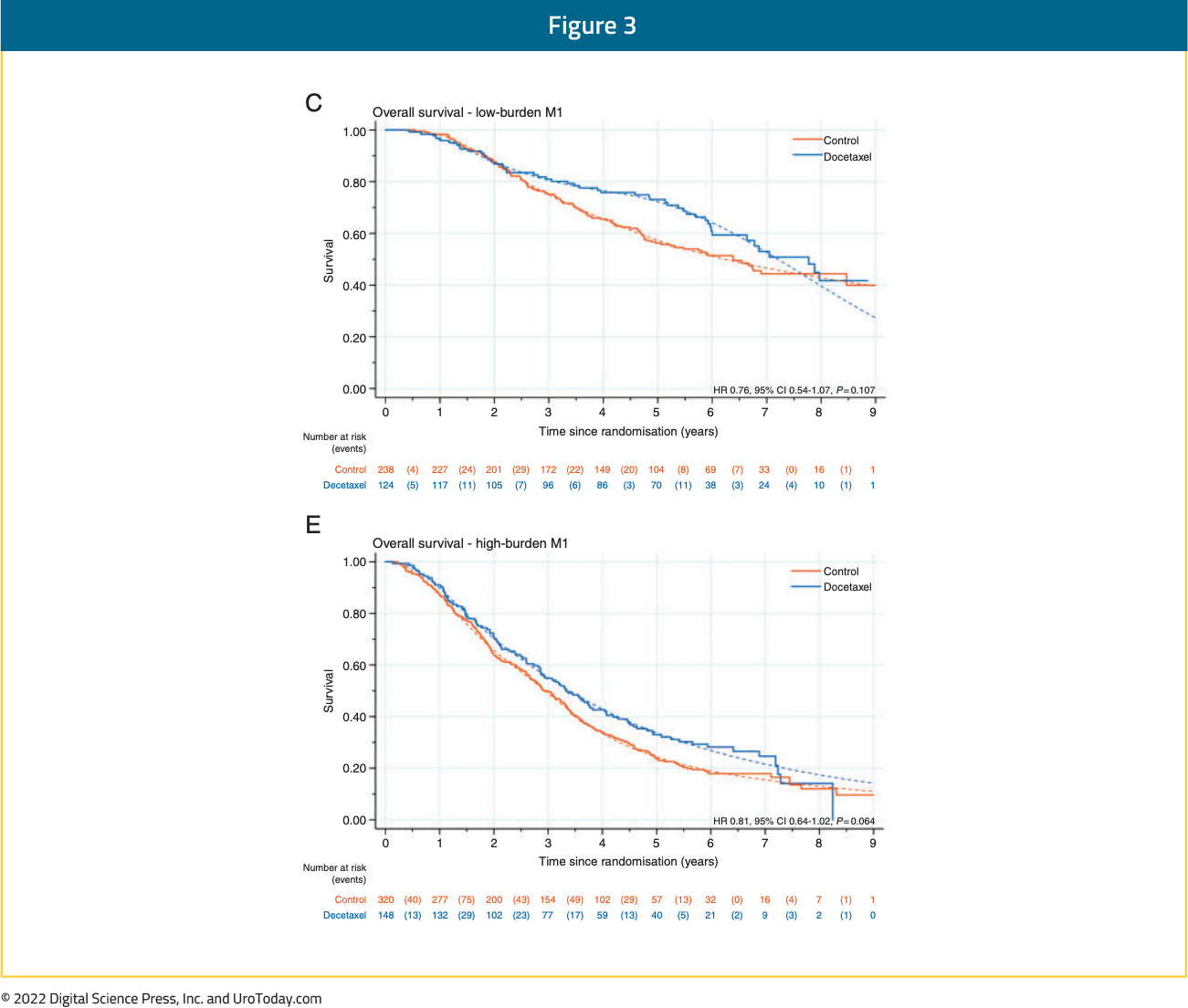
At ASCO 2022, the results of a STOPCAP M1 collaborative meta-analysis of individual patient data from GETUG-15, STAMPEDE, and CHAARTED was presented. This meta-analysis included all 2,261 randomized patients, with median follow-up of 6 years. There were clear benefits for docetaxel on OS (HR 0.79, 95% CI 0.70 to 0.88), progression-free survival (PFS) (HR 0.70, 95% CI 0.63 to 0.77) and failure-free survival (FFS) (HR 0.64, 95% CI 0.58 to 0.71) in the overall pooled cohort. With evidence of non-proportional hazards, the estimated 5-year absolute differences were: OS 11% (95% CI 6 to 15%), PFS 9% (95% CI 5 to 13%) and FFS 9% (95% CI 6 to 12%).
Notably, the relative effect of docetaxel on PFS differed by volume of metastases (interaction p=0.027; high volume HR 0.60, 95% CI 0.52 to 0.68; low volume HR 0.78, 95% CI 0.64 to 0.94) and timing of metastatic disease (interaction p=0.077; synchronous HR 0.67, 95% CI 0.60 to 0.75; metachronous HR 0.89, 95% CI 0.67 to 1.18). OS results were similar. When metastatic disease volume and timing were combined, docetaxel appeared to improve PFS and OS for all men, except those with low volume, metachronous disease, though there is a clear dose-response relationship with a diminishing benefit to docetaxel chemotherapy from high-volume synchronous disease, to high-volume metachronous disease, to low-volume synchronous disease, and finally to low-volume, metachronous disease:
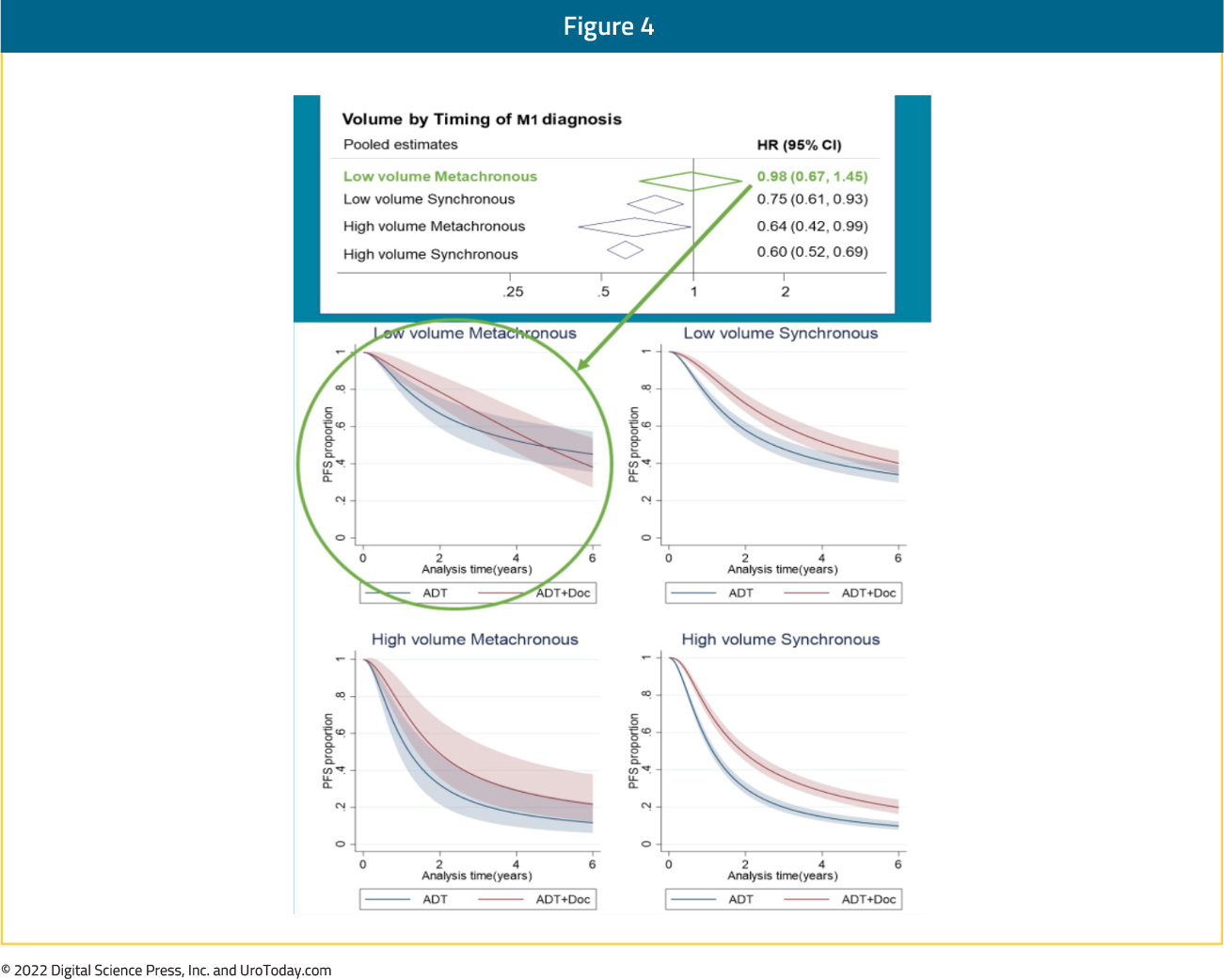
Taken together, it appears that docetaxel addition has a clear OS benefit in patients with high volume disease, irrespective of timing of disease (synchronous or metachronous). Patients with synchronous low-volume disease may have a modest benefit with docetaxel addition though those with low volume, metachronous (i.e. recurrent) mHSPC do not benefit from docetaxel addition to ADT.
ADT + Abiraterone/Prednisone
The LATITUDE trial published in 2017 included only patients with de novo, high risk prostate cancer, leading to the LATITUDE high risk criteria (as opposed to high versus low volume), defined as patients with two or more of the following characteristics: (i) Gleason Score ≥8, (ii) presence of ≥3 lesions on bone scan, and (iii) presence of measurable visceral lesions. These risk criteria were applied in a post hoc subgroup analysis of the 2017 STAMPEDE “abiraterone comparison”.10Among 990 patients, 901 patients with available data were included. LATITUDE high-risk disease was present in 52% while the remainder had so-called low-risk disease. Overall survival benefits with ADT + abiraterone/prednisone addition were seen in both the low (HR 0.66, 95% CI 0.44 to 0.98) and high-risk groups (HR 0.54, 95% CI 0.41 to 0.70). The heterogeneity of treatment effect between high- and low-risk groups was not statistically significant (p-interaction = 0.39). When the CHAARTED criteria were applied in this same cohort, consistent OS benefits were again seen in both the low- (HR 0.64, 95% CI 0.42 to 0.97) and high-volume groups (HR 0.60, 95% CI 0.46 to 0.78).
ADT + Apalutamide vs ADT
In the TITAN trial, 81% of patients presented with de novo mHSPC and 62.7% of patients had CHAARTED high volume disease.11 A subgroup analysis from TITAN demonstrated that addition of apalutamide maintained OS benefits irrespective of:Disease volume
- High: HR: 0.70 (95% CI: 0.56-0.88)
- Low: HR: 0.52 (95% CI: 0.35-0.79)
- Synchronous: HR: 0.68 (95% CI: 0.55-0.85)
- Metachronous: HR: 0.39 (95% CI: 0.22-0.69)
ADT + Enzalutamide vs ADT
In 2021, Sweeney et al. performed subgroup analyses of patients with metachronous mHSPC treated in the ENZAMET trial of enzalutamide. Of the 1,125 enrolled patients, 312 (28%) had known metachronous disease and 205 (66%) had low-volume disease at entry. For the metachronous mHSPC group overall, OS HR was 0.56 (95% CI 0.29 to 1.06) for enzalutamide addition. Interestingly, this OS benefit seemed to be driven mainly by benefits in the low-volume subgroup (HR 0.40, 95% CI 0.16 to 0.97) with no apparent benefit in the high volume metachronous subgroup (HR 0.86, 95% CI 0.33 to 2.22). These results may have been secondary to the high use of concurrent docetaxel in the high-volume subgroup (60% vs 15% in patients with low-volume disease), which may have diluted an OS benefit in the high volume subgroup.12Most recently at ASCO 2022, Dr. Ian Davis presented the most recent update of the ENZAMET trial. With regards to subgroup analyses, enzalutamide demonstrated consistent OS benefits across the following strata:
Planned early docetaxel (p-value for interaction=0.09)
- Yes: HR 0.82 (95% CI 0.63 to 1.06)
- No: HR 0.60 (95% CI 0.47 to 0.78)
- Low: HR 0.54 (95% CI 0.39 to 0.74)
- High: HR 0.79 (95% CI 0.63 to 0.98)
- Synchronous: HR 0.70 (95% CI 0.56 to 0.87)
- Metachronous: HR 0.71 (95% CI 0.52 to 0.98)
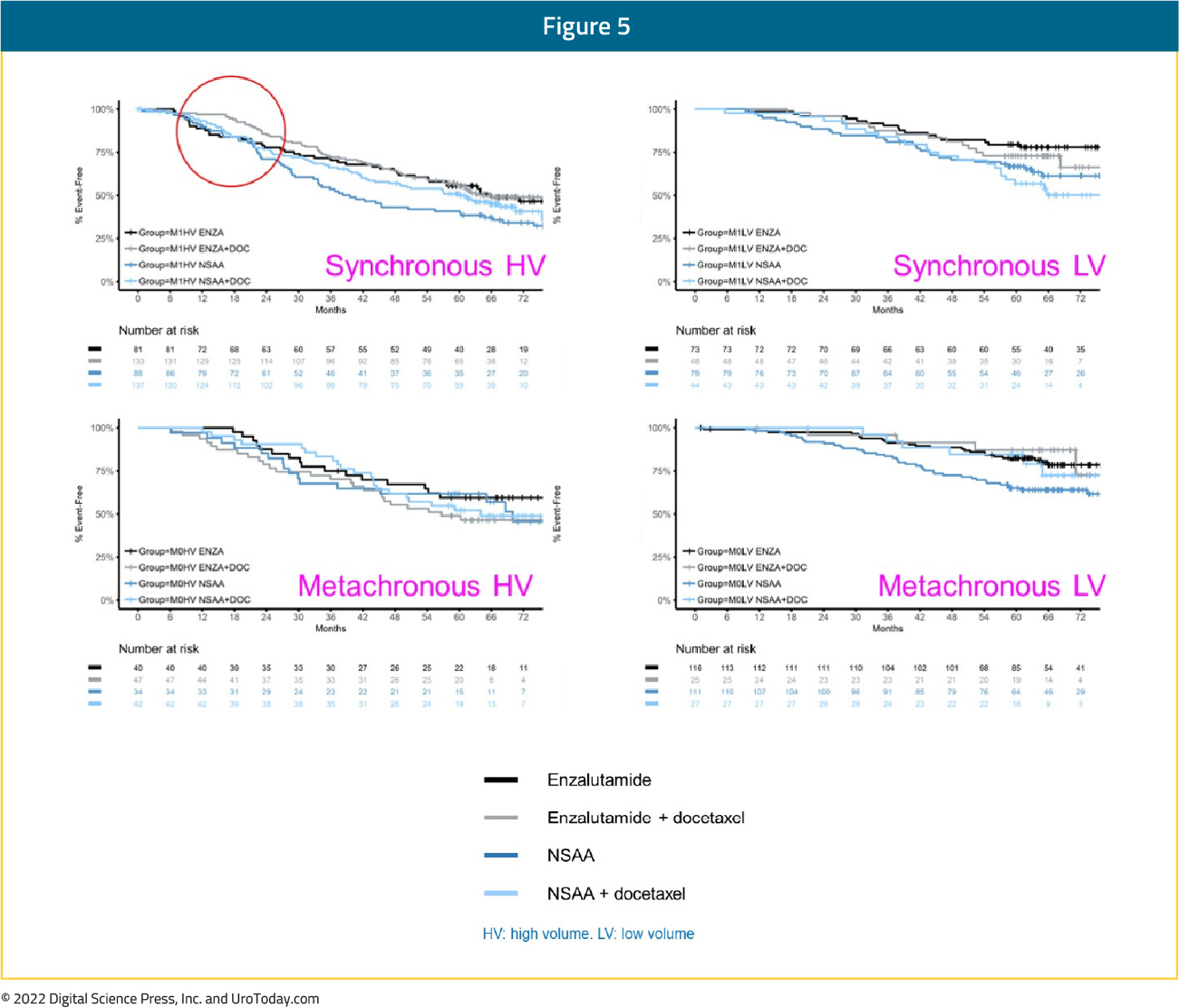
In the ARCHES trial, patients with predominately high-volume (63%) and de novo (66.7%) disease were randomized to enzalutamide + ADT or ADT alone. Notably, 17% of the cohort had previously received docetaxel.13 The most recent update of the trial was presented at ASCO GU 2022, noting that there were consistent benefits for the use of enzalutamide in addition to ADT in all disease volume and M0/M1 populations:
- Synchronous, high volume: HR 0.63 (95% CI 0.48 to 0.81)
- Synchronous, low volume: HR 0.65 (95% CI 0.39 to 1.08)
- Metachronous, high volume: HR 0.77 (95% CI 0.39 to 1.50)
- Metachronous, low volume: HR 0.63 (95% CI: 0.26 to 1.54)
ADT + Docetaxel + Abiraterone
Initially presented at ASCO 2021 and subsequently published in the Lancet in 2022,22 the PEACE-1 trial employed a 2x2 design to assess, (separately and combined) the impact of the addition of abiraterone + prednisone + ADT and radiation therapy to standard of care therapy in men with de novo mHSPC. Among patients with high volume disease, the addition of abiraterone + prednisone + ADT to standard of care resulted in a 53% improvement in rPFS with median rPFS of 1.6 years on the standard of care arm and 4.1 years on the standard of care plus abiraterone + prednisone + ADT arm (HR 0.47, 95% CI 0.36 to 0.60). The addition of abiraterone + prednisone + ADT to standard of care in patients with low volume disease resulted in a 42% improvement in rPFS with median rPFS of 2.7 years on the standard of care arm versus not yet reached on the standard of care plus abiraterone + prednisone + ADT arm (HR 0.58, 95% CI 0.39-0.87). With regards to OS, this effect was seen across subgroups, including those with high volume disease (HR 0.72, 95% CI 0.55 to 0.95) and low volume disease (HR 0.83, 95% CI 0.50 to 1.38; interaction P-value 0.64). Notably, the OS data is immature for the low volume patients due to a small number of events.ADT + Docetaxel + Darolutamide
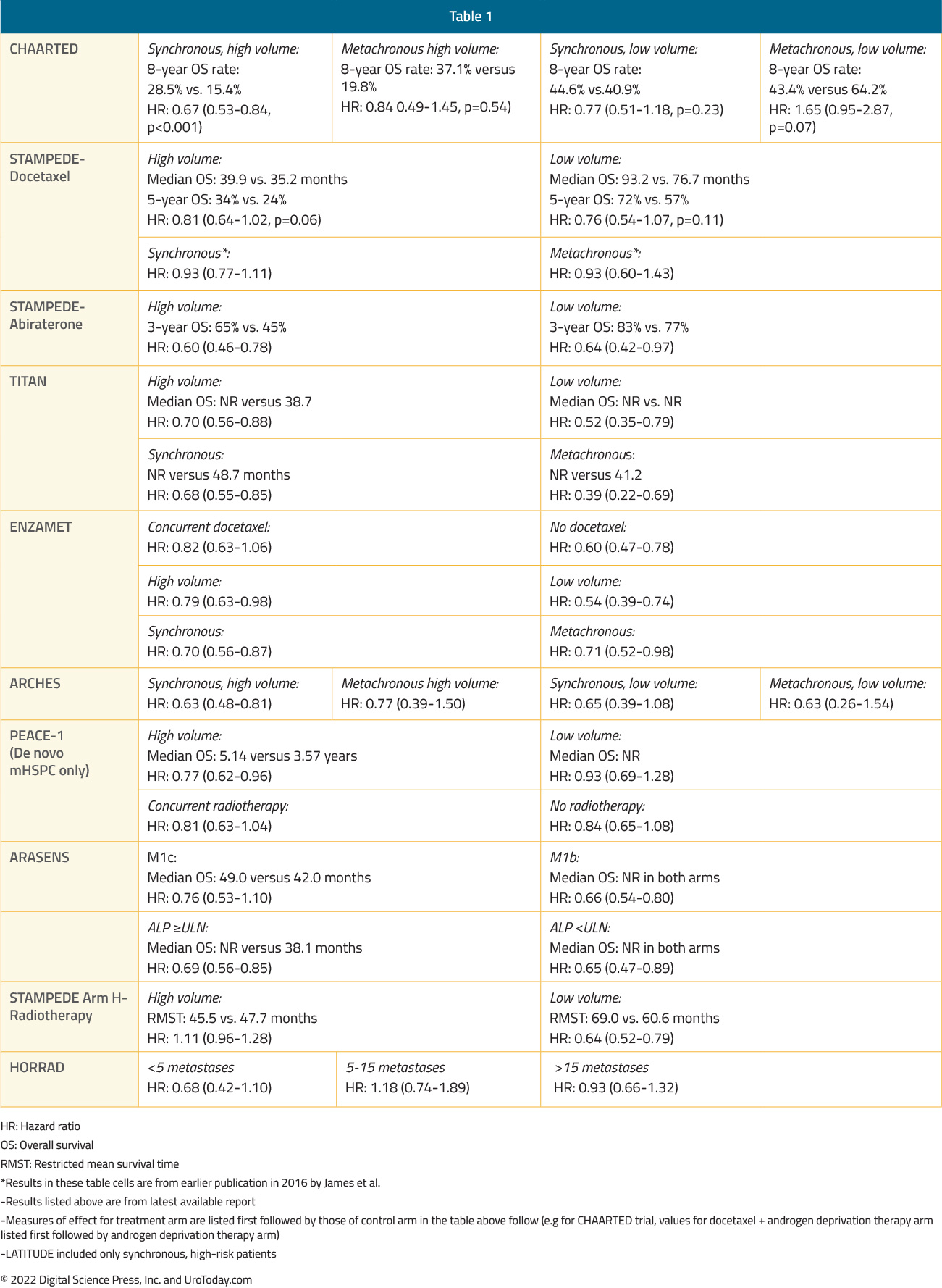
The ARASENS trial evaluating addition of darolutamide to standard of care therapy consisting of ADT + docetaxel was presented at ASCO GU 2022 and concurrently published in The New England Journal of Medicine.14 Metastatic burden classification by CHAARTED criteria was not available in this trial, however disease stratification by TNM metastatic burden (M1b versus M1c) demonstrated consistent benefits for darolutamide addition to ADT and docetaxel. In the M1b subgroup (with bony metastases), HR for OS was 0.66 (95% CI 0.54 to 0.80). In the M1c group (with visceral metastases), HR for OS was 0.76 (95% CI 0.53 to 1.10), with median OS of 49.0 months in the darolutamide arm and 42.0 months in the placebo arm.The following table summarizes OS outcomes by CHAARTED disease volume criteria and presentation (synchronous vs metachronous) among men with mHSPC:
Specific Considerations for Low Volume mHSPC Patients
Beyond systemic treatment intensification, there is a role for therapy targeting either the primary (for patients with de novo disease) or metastatic sites in certain patient groups.Prostate radiotherapy: STAMPEDE (Arm H) was an open label, randomized controlled phase III trial of 2,061 men with de novo mHSPC randomized to standard of care + radiotherapy or standard of care. Subgroup analysis by metastatic volume (CHAARTED criteria) was planned a priori. Radiotherapy when stratified by metastatic burden showed an OS benefit in the low volume group (HR 0.68, 95% CI 0.52 to 0.90) with restricted mean survival time improved by 3.6 months from 45.4 to 49.1.15 Updated results of this trial were published June 2022 in PLoS Medicine.16 With a median follow up of 61.3 months, prostate radiotherapy continued to demonstrate OS benefits in patients with low metastatic burden (HR 0.64, 95% CI 0.52 to 0.79). No benefit was seen in patients with high metastatic burden (HR 1.11, 95% CI 0.96 to 1.28; interaction p=0.001).
HORRAD was a multicenter trial of 432 patients with previously untreated, de novo mHSPC men randomized in a 1:1 fashion to either ADT with external beam radiotherapy or ADT alone. No subgroup analyses by CHAARTED volume criteria was performed, but subgroup analysis by number of metastatic lesions suggested potential (albeit not statistically significant) OS benefit for radiotherapy in patients with <5 metastatic sites (HR 0.68, 95% CI 0.42 to 1.10).17
In 2019, a systematic review and meta-analysis of the STAMPEDE Arm H and HORRAD trials was performed by the STOPCAP collaboration. Pooled results of 2,126 men demonstrated no overall OS improvement (HR 0.92, 95% CI 0.81 to 1.04) or PFS (HR 0.94, 95% CI to 0.84-1.05). However, the effect of prostate radiotherapy on OS varied by metastatic burden (<5 versus ≥5 bone metastases: interaction HR 1.47, 95% CI 1.11 to 1.94, p=0.007). Furthermore, there was a 7% improvement in 3-yr survival in men with fewer than five bone metastases.18
Metastasis-Directed Therapy: In addition to systemic treatment intensification and local prostate-directed radiotherapy, treatment may be intensified by targeting local treatment to sites of metastatic disease. To date, there is no level one evidence supporting MDT in synchronous oligometastatic mHSPC disease space. Three randomized trials to date have evaluated MDT (stereotactic body radiotherapy or surgical metastasectomy) in patients with metachronous, oligometastatic mHSPC.
The STOMP trial was a multicenter, randomized phase II trial that prospectively evaluated the effects of MDT for patients with evidence of oligometastatic disease on choline PET/CT (up to three extracranial sites) who had received prior treatment with curative intent and had evidence of biochemical recurrence with testosterone >50 ng/ml (i.e. metachronous, oligometastatic mHSPC). Between 2012 and 2015, 62 patients were randomized 1:1 and MDT was either SBRT or metastasectomy. The primary endpoint was time to initiation of ADT (called ADT-free survival). ADT was initiated for symptoms, progression beyond three metastases, or local progression of known metastatic disease. Time to castration resistance was a secondary endpoint (called CRPC-free survival). The updated five-year results were presented at GU ASCO 2020. With a median follow up of 5.3 years, the five-year ADT-free survival was 8% in the surveillance arm compared to 34% for the MDT group (HR 0.57, 95% CI 0.38 to 0.84, log-rank p=0.06). No differences were seen between groups when stratified by nodal versus non-nodal metastases. Secondary endpoint of CRPC-free survival at 5 years was 53% in subjects under surveillance and 76% in those receiving MDT (HR 0.62, 80% CI 0.35 to 1.09).19
The ORIOLE trial was a randomized phase II trial of 54 men with metachronous, oligometastatic mHSPC (up to three sites). Metastatic sites were diagnosed via 18F-DCFPyL PET/CT. Between 2016 and 2018, patients were randomized in a 2:1 fashion to receive SABR or observation. The primary outcome was progression at 6 months, defined as serum PSA increase, progression detected by conventional imaging, symptomatic progression, ADT initiation for any reason, or death. Progression at six months occurred in 7 of 36 patients (19%) receiving SABR and 11 of 18 patients (61%) undergoing observation (p = 0.005). Treatment with SABR improved median PFS (not reached vs 5.8 months; HR 0.30, 95% CI 0.11 to 0.81). No toxic effects of grade 3 or greater were observed.20
SABR-COMET was a randomized, open-label phase II study of patients with oligometastatic disease (up to five sites) between February 2012 and August 2016. This trial was not restricted to patients with prostate cancer and also included lung, breast, and colorectal cancer patients. Of the 99 patients in this trial, 18 (18%) had prostate cancer. After stratifying by the number of metastases (1–3 vs 4–5), patients were randomized in a 1:2 fashion to receive either palliative standard of care alone or standard of care plus SABR. In an updated analysis published in 2020 (median follow up 51 months), the five-year OS rate was 17.7% (95% CI 6-34%) in the control arm and 42.3% in the SABR arm (95% CI 28-56%, stratified log-rank p=0.006). The corresponding median OS was 28 months and 50 months, respectively. There were no new grade 2-5 adverse events and no differences in QOL between the arms.21,22
Synthesizing the Timing of Metastasis and Volume of Disease Relationship
In clinical practice, the two faces of mHSPC may be very different. Dr. Chris Sweeney has lectured at length regarding the differences between de novo mHSPC (ie. a 55-year-old with no comorbidities and high volume de novo/synchronous metastatic disease) and metachronous mHSPC (ie. an 82-year-old with congestive heart failure and coronary artery disease with 2 bone metastases 10 years after prostatectomy). As highlighted above, when assessing the 8-year OS CHAARTED data, the addition of docetaxel to ADT shows a clear survival benefit for those with de novo high volume disease and those with metachronous high volume disease, a modest effect for those with synchronous low volume disease, and no benefit for those with metachronous low volume disease, men with biochemical relapse, and in the adjuvant setting. Alternatively, this is not the case for second generation anti-androgens: enzalutamide + ADT in ENZAMET showed improved PFS and OS among all subgroups (synchronous high-volume, synchronous low-volume, metachronous high-volume, and metachronous low-volume). This is also true in the ARCHES, TITAN, LATITUDE and STAMPEDE-abiraterone trials. Interestingly, subgroup analyses from ENZAMET (which permitted docetaxel use), demonstrate that docetaxel addition to enzalutamide + ADT only shows a survival benefit in the poorest prognosis group of men with synchronous, high volume disease; these findings are similar to those reported PEACE-1 and ARASENS. As such, it is important when developing a mHSPC treatment plan to consider the presentation and distribution of metastases, and the available options for ADT + a second/third agent: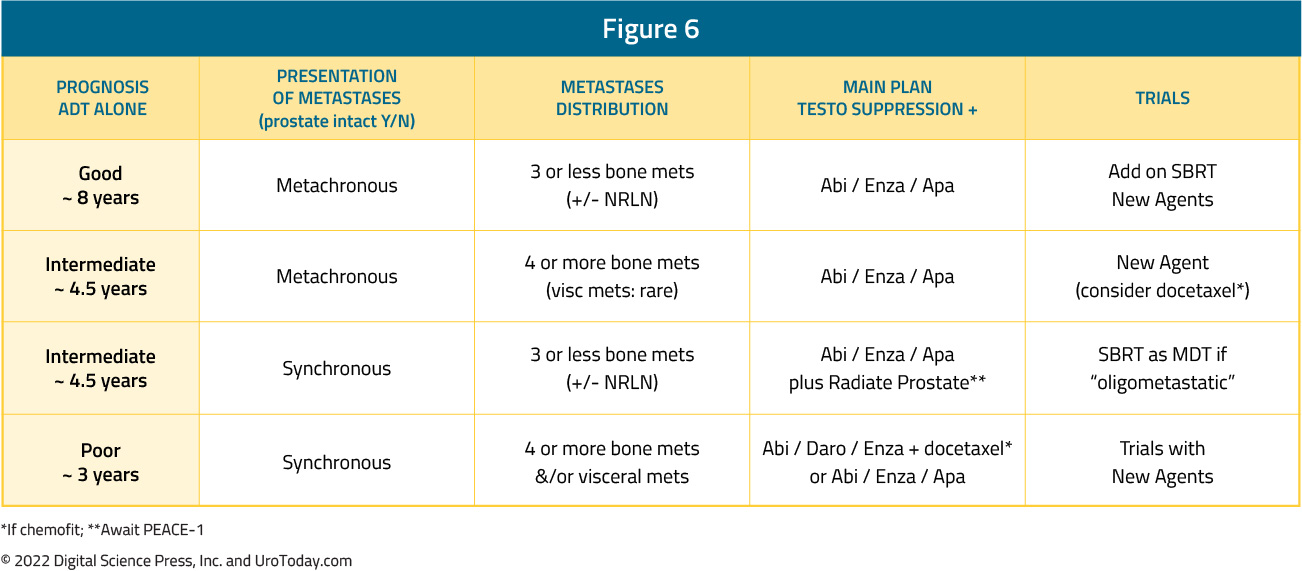
- Written by: Rashid K. Sayyid, MD, MSc, and Zachary Klaassen, MD, MSc
- References:
- Cancer Stat Facts: Prostate Cancer. National Cancer Institute. Available at https://seer.cancer.gov/statfacts/html/prost.html. Accessed: July 17, 2022.
- Weiner AB, Siebert AL, Fenton SE, et al. First-line Systemic Treatment of Recurrent Prostate Cancer After Primary or Salvage Local Therapy: A Systematic Review of the Literature. Eur Urol Oncol. 2022.
- Deek MP, Van der Eecken K, Phillips R, et al. The mutational landscape of metastatic castration-sensitive prostate cancer: the spectrum theory revisited. Eur Urol. 2021;80:632-640
- Stopsack KH, Nandakumar S, Wimber AG, et al. Oncogenic genomic alterations, clinical phenotypes, and outcomes in metastatic castration-sensitive prostate cancer. Clin Cancer Res. 2020;26:3230-3238.
- Sweeney CJ, Chen Y, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N ENgl J Med. 2015;373:737-746.
- Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J Clin Oncol. 2018;36(11):1080-1087.
- Marino P, Sfumato P, Joly F, et al. Q-TWiST analysis of patients with metastatic castrate naive prostate cancer treated by androgen deprivation therapy with or without docetaxel in the randomised phase III GETUG-AFU 15 trial. Eur J Cancer. 2017;84:27-33.
- Gravis G, Boher J, Chen Y, et al. Burden of Metastatic Castrate Naive Prostate Cancer Patients, to Identify Men More Likely to Benefit from Early Docetaxel: Further Analyses of CHAARTED and GETUG-AFU15 Studies. Eur Urol. 2018;73(6):847-855.
- Clarke NW, Ali A, Ingleby FC, et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results from the STAMPEDE trial. Ann Oncol. 2019;30(12):1992-2003.
- Hoyle AP, Ali A, James ND, et al. Abiraterone in “High-” and “Low-risk” Metastatic Hormone-sensitive Prostate Cancer. Eur Urol. 2018;76(6):719-728.
- Chi KN, Chowdhury S, Bjartell A, et al. Apalutamide in Patients With Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J Clin Oncol. 2021;39(2):2294-2303.
- Sweeney CJ, Martin AJ, Stockler MR, et al. Overall survival of men with metachronous metastatic hormone-sensitive prostate cancer treated with enzalutamide and androgen deprivation therapy. Eur Urol. 2021;80:275-279.
- Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol. 2019;37(32):2974-2986.
- Chi KN, Chowdhury S, Bjartell A, et al. Apalutamide in Patients With Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J Clin Oncol. 2021;39(2):2294-2303.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353-2366.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the prostate for men with metastatic prostate cancer in the UK and Switzerland: Long-term results from the STAMPEDE randomised controlled trial. PLoS Medicine. 2022;19(6):e1003998.
- Boeve LMS, Hulshof MCCM, Vis AN, et al. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. Eur Urol. 2019;75(3):410-418.
- Burdett S, Boeve LM, Ingleby FC, et al. Prostate Radiotherapy for Metastatic Hormone-sensitive Prostate Cancer: A STOPCAP Systematic Review and Meta-analysis. Eur Urol. 2019;76(1):115-124.
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: A prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36(5):446-453.
- Phillips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate CancerThe ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020;6(5):650-659.
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185):2051-2058.
- Palma DA, Olson R, Harrow S, et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J Clin Oncol. 2020;38(25):2830-2838.
PSMA PET Imaging in Prostate Cancer: Staging for Unfavorable Intermediate and High-Risk Disease
- Written by: Rashid Sayyid, MD MSc, & Zachary Klaassen, MD MSc
- References:
- Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395(10231):1208-1216.
- De Feria Cardet RE, Hofman MS, Segard T, et a. Is Prostate-specific Membrane Antigen Positron Emission Tomography/Computed Tomography Imaging Cost-effective in Prostate Cancer: An Analysis Informed by the proPSMA Trial. Eur Urol. 2021;79(3):413-8.
- Hope TA, Eiber M, Armstrong WR, et al. Diagnostic Accuracy of 68Ga-PSMA-11 PET for Pelvic Nodal Metastasis Detection Prior to Radical Prostatectomy and Pelvic Lymph Node Dissection: A Multicenter Prospective Phase 3 Imaging Trial. JAMA Oncol. 2021;7(11):1635-42.
- Van Kalmthout LWM, van Melick HHE, Lavalaye J, et al. Prospective Validation of Gallium-68 Prostate Specific Membrane Antigen-Positron Emission Tomography/Computerized Tomography for Primary Staging of Prostate Cancer. J Urol. 2020;203(3):537-45.
- Pienta KJ, Gorin MA, Rowe SP, et al. A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18 F-DCFPyL in Prostate Cancer Patients (OSPREY). J Urol 2021;206(1):52-61.
- Jansen BHE, Bodar YJL, Zwezerijnen GJC, et al. Pelvic lymph-node staging with 18F-DCFPyL PET/CT prior to extended pelvic lymph-node dissection in primary prostate cancer - the SALT trial. Eur J Nucl Med Mol Imaging. 2021;48(2):509-20.
- Prostate Cancer. https://uroweb.org/guidelines/prostate-cancer. Accessed on Aug 14, 2022
- Prostate Cancer. NCCN Clinical Practice Guidelines in Oncology. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed on August 27, 2022.
The Current Landscape of Metastatic Hormone Sensitive Prostate Cancer: Treatment Utilization and Future Directions
Introduction
The treatment landscape of advanced prostate cancer continues to evolve, particularly over the last 5+ years. Although there are several treatment options, including both ADT-based doublet and triplet combinations, available for men with metastatic hormone sensitive prostate cancer (mHSPC) that have showed an OS benefit versus ADT alone, there remain several unaddressed questions. - Written by: Rashid K. Sayyid, MD, MSc & Zachary Klaassen, MD, MSc
- References:
- Mori K, Mostafaei H, Motlagh RS, et al. Systemic therapies for metastatic hormone-sensitive prostate cancer: network meta-analysis. BJU Int. 2021;12(4):423-433.
- Wang L, Paller CJ, Hong H, et al. Comparison of Systemic Treatments for Metastatic Castration-Sensitive Prostate CancerA Systematic Review and Network Meta-analysis. JAMA Oncol. 2021;7(3):412-420.
- Sathianathen NJ, Koschel S, Thangasamy IA, et al. Indirect Comparisons of Efficacy between Combination Approaches in Metastatic Hormone-sensitive Prostate Cancer: A Systematic Review and Network Meta-analysis. Eur Urol. 2020;77(3):365-372.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353-2366.
- Wallis CJD, Malone S, Cagiannos I, et al. Real-World Use of Androgen-Deprivation Therapy: Intensification Among Older Canadian Men With de Novo Metastatic Prostate Cancer. JNCI Cancer Spectr. 2021;5(6):pkab082.
- Swami U, Sinnott JA, Haaland B, et al. Treatment Pattern and Outcomes with Systemic Therapy in Men with Metastatic Prostate Cancer in the Real-World Patients in the United States. Cancers (Basel). 2021;13(19):4951.
- Sartor O, de Bono J, Chi KN, et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021;385:1091-1103.
- Hofman MS, Emmett L, Sandhu S, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397(10276):797-804.
- de Bono JS, Mehra N, Scagliotti GV, et al. Talazoparib monotherapy in metastatic castration-resistant prostate cancer with DNA repair alterations (TALAPRO-1): An open-label, phase 2 trial. Lancet Oncol. 2021;22(9):1250-1264.
The Current Landscape of PSMA PET Imaging in Prostate Cancer: Advanced Prostate Cancer
- Written by: Rashid Sayyid, MD MSc, & Zachary Klaassen, MD MSc
- References:
- Deek MP, van der Eecken K, Sutera P, et al. Long-Term Outcomes and Genetic Predictors of Response to Metastasis-Directed Therapy Versus Observation in Oligometastatic Prostate Cancer: Analysis of STOMP and ORIOLE Trials. J Clin Oncol. 2022;JCO2200644.
- Philips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020;6(5):650-9.
- Kneebone A, Hruby G, Ainsworth H, et al. Stereotactic Body Radiotherapy for Oligometastatic Prostate Cancer Detected via Prostate-specific Membrane Antigen Positron Emission Tomography. Eur Urol Oncol. 2018;1(6):531-7.
- Fizazi K, Shore N, Tammela TL, et al. Nonmetastatic, Castration-Resistant Prostate Cancer and Survival with Darolutamide. N Engl J Med. 2020;383:1040–9.
- Smith MR, Saad F, Chowdhury S, et al. Apalutamide and Overall Survival in Prostate Cancer. Eur Urol. 2021;79:150–8.
- Sternberg CN, Fizazi K, Saad F, et al. Enzalutamide and Survival in Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med. 2020;382:2197–206.
- Fendler WP, Weber M, Iravani A, et al. Prostate-Specific Membrane Antigen Ligand Positron Emission Tomography in Men with Nonmetastatic Castration-Resistant Prostate Cancer. Clin Cancer Res. 2019;25(24):7448-54.
- Wang B, Liu C, Wei Y, et al. A Prospective Trial of 68Ga-PSMA and 18F-FDG PET/CT in Nonmetastatic Prostate Cancer Patients with an Early PSA Progression During Castration. Clin Cancer Res. 2020;26(17):4551-8.
- Fourquet A, Aveline C, Cussenot O, et al. 68 Ga-PSMA-11 PET/CT in restaging castration-resistant nonmetastatic prostate cancer: detection rate, impact on patients' disease management and adequacy of impact. Sci Rep. 2020;10(1):2104.
- Wright GL, Grob BM, Haley C, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48:326–34.
- Evans MJ, Smith-Jones PM, Wongvipat J, et al. Noninvasive measurement of androgen receptor signaling with a positron-emitting radiopharmaceutical that targets prostate-specific membrane antigen. Proc Natl Acad Sci USA. 2011;108:9578–82.
- Aggarwal R, Wei X, Kim W, et a. Heterogeneous Flare in Prostate-specific Membrane Antigen Positron Emission Tomography Tracer Uptake with Initiation of Androgen Pathway Blockade in Metastatic Prostate Cancer. Eur Urol Oncol. 2018;1(1):78-82.
- Emmett L, Yin C, Crumbaker M, et al. Rapid Modulation of PSMA Expression by Androgen Deprivation: Serial 68Ga-PSMA-11 PET in Men with Hormone-Sensitive and Castrate-Resistant Prostate Cancer Commencing Androgen Blockade. J Nucl Med. 2019;60:950-4.
- Ettala O, Malaspina S, Tuokkola T, et al. Prospective study on the effect of shortterm androgen deprivation therapy on PSMA uptake evaluated with 68Ga-PSMA-11 PET/MRI in men with treatment-naïve prostate cancer. Eur J Nucl Med Mol Imaging. 2020;47:665–673.
- Afshar-Oromieh A, Debus N, Uhrig M, et al. Impact of long-term androgen deprivation therapy on PSMA ligand PET/CT in patients with castration-sensitive prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:2045–2054.
- Seitz AK, Rauscher I, Haller B, et al. Preliminary results on response assessment using 68Ga-HBED-CC-PSMA PET/CT in patients with metastatic prostate cancer undergoing docetaxel chemotherapy. Eur J Nucl Med Mol Imaging 2018;45:602–12.
- Grubmüller B, Razul S, Baltzer P, et al. Response assessment using [68Ga]Ga-PSMA ligand PET in patients undergoing systemic therapy for metastatic castration-resistant prostate cancer. Prostate 2020;80:74–82.
- Kallur KG, Ramachandra PG, Rajkumar K, et al. Clinical utility of gallium-68 PSMA PET/CT scan for prostate cancer. Indian J Nucl Med 2017;32:110–7.
- Fanti S, Hadaschik B, Herrmann K. Proposal for Systemic-Therapy Response-Assessment Criteria at the Time of PSMA PET/CT Imaging: The PSMA PET Progression Criteria. J Nucl Med. 2020;61(5):678-82.
The Current Landscape of Metastatic Hormone Sensitive Prostate Cancer: A Plethora of Treatment Options
Introduction
The treatment landscape of metastatic hormone sensitive prostate cancer (mHSPC) has evolved rapidly since the introduction of combination chemohormonal therapy with docetaxel and androgen deprivation therapy (ADT) in 2015.1 This includes a variety of treatment intensification strategies including both systemic therapy and local, prostate-directed radiotherapy. In addition to docetaxel, there are several U.S. Food and Drug Administration (FDA) approved agents in this disease space in combination with ADT:- Written by: Zachary Klaassen, MD, MSc and Rashid K. Sayyid, MD, MSc
- References:
- Sweeney CJ, Chen Y, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N ENgl J Med. 2015;373:737-746.
- Gravis G, Fizazi K, Joly F, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(2):149-158.
- Sweeney CJ, Chen Y, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N ENgl J Med. 2015;373:737-746.
- James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163-1177.
- Tucci M, Bertaglia V, Vignani F, et al. Addition of Docetaxel to Androgen Deprivation Therapy for Patients with Hormone-sensitive Metastatic Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol. 2016;69(4):563-573.
- Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J Clin Oncol. 2018;36(11):1080-1087.
- Clarke NW, Ali A, Ingleby FC, et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results from the STAMPEDE trial. Ann Oncol. 2019;30(12):1992-2003.
- Fizazi K, Tran N, Fein L, et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. New Engl J Med.. 2017;377(4):352-360.
- Hoyle AP, Ali A, James ND, et al. Abiraterone in “High-” and “Low-risk” Metastatic Hormone-sensitive Prostate Cancer. Eur Urol. 2018;76(6):719-728.
- James N, Clarke NW, Cook A, et al. Abirtaterone acetate plus prednisolone for metastatic patients starting hormone therapy: 5-year followup results from the STAMPEDE randomized trial. Int J Cancer. 2022;151(3):422-434.
- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381(1):13-24.
- Chi KN, Chowdhury S, Bjartell A, et al. Apalutamide in Patients With Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J Clin Oncol. 2021;39(2):2294-2303.
- Agarwal N, McQuarrie K, Bjartell A, et al. Health-related quality of life after apalutamide treatment in patients with metastatic castration-sensitive prostate cancer (TITAN): a randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2019;20(11):1518-1530.
- Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med. 2019;381(2):121-131.
- Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol. 2019;37(32):2974-2986.
- Fizai K, Foulon S, Carles J, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet 2022;399(10336):1695-1707.
- Smith MR, Hussain M, Saad F, et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med. 2022;386(12):1132-1142.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353-2366.
- Boeve LMS, Hulshof MCCM, Vis AN, et al. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. Eur Urol. 2019;75(3):410-418.
The Current Landscape of PSMA PET Imaging in Prostate Cancer: Evaluating Men with Biochemical Recurrence
Although definitive local therapy in the form of radical prostatectomy or radiation therapy with or without ADT offers excellent long-term outcomes for the majority of patients with clinically localized prostate cancer, patients with high-risk disease experience primary treatment failure rates approaching 65%.1 Disease persistence/recurrence in such patients may be restricted to the prostatic fossa, pelvic lymph nodes, non-regional lymph nodes (M1a), bones (M1b), or the viscera (M1c).
- Written by: Rashid Sayyid, MD MSc, & Zachary Klaassen, MD MSc
- References:
- D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969-74.
- Jadvar H. Is There Utility for FDG PET in Prosate Cancer? Semin Nucl Med. 2016;46(6):502-506.
- Picchio M, Briganti A, Fanti S, et al. The role of choline positron emission tomography/computed tomography in the management of patients with prostate-specific antigen progression after radical treatment of prostate cancer. Eur Urol 2011;59:51-60.
- Krause BJ, Souvatzoglou M, Tuncel M, et al. The detection rate of [11C]choline-PET/CT depends on the serum PSA-value in patients with biochemical recurrence of prostate cancer. Eur J Nucl Med Mol Imaging 2008;35:18-23.
- Rayn KN, Elnabawi YA, Sheth N. Clinical implications of PET/CT in prostate cancer management. Transl Androl Urol. 2018;7(5):844-54.
- Afshar-Oromieh A, Malcher A, Eder M, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging 2013;40:486-95.
- Morigi JJ, Stricker PD, van Leewen PJ, et al. Prospective Comparison of 18F-Fluoromethylcholine Versus 68Ga-PSMA PET/CT in Prostate Cancer Patients Who Have Rising PSA After Curative Treatment and Are Being Considered for Targeted Therapy. J Nucl Med. 2015;56(8):1185-90.
- Calais J, Ceci F, Eiber M, et al. 18F-fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019;20(9):1286-94.
- Fendler WP, Calais J, Eiber M, et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer. JAMA Oncol. 2019; 5(6):856-63.
- Caroli P, Sandler I, Matteucci F, et al. 68 Ga-PSMA PET/CT in patients with recurrent prostate cancer after radical treatment: prospective results in 314 patients. Eur J Nucl Med Mol Imaging. 2018;45(12):2035-44.
- Ceci F, Castellucci P, Graziani T, et al. 68Ga-PSMA-11 PET/CT in recurrent prostate cancer: efficacy in different clinical stages of PSA failure after radical therapy. Eur J Nucl Med. 2019;46:31-39.
- Mazrani W, Cook GJR, Bomanji J. Role of 68Ga and 18F PSMA PET/CT and PET/MRI in biochemical recurrence of prostate cancer: a systematic review of prospective studies. Nucl Med Commun. 2022;43(6):631-7.
- Morris MJ, Rowe SP, Gorin MA, et al. Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase 3, Multicenter Study. Clin Cancer Res. 2021;27(13):3674-82.
- Ong S, Pascoe C, Kelly BD, et al. PSMA PET-CT Imaging Predicts Treatment Progression in Men with Biochemically Recurrent Prostate Cancer-A Prospective Study of Men with 3 Year Follow Up. Cancers (Basel). 2022;14(11):2717.
- Emmett L , Tang R, Nandrukar R, et al. 3-Year Freedom from Progression After 68 Ga-PSMA PET/CT-Triaged Management in Men with Biochemical Recurrence After Radical Prostatectomy: Results of a Prospective Multicenter Trial. J Nucl Med. 2020;61(6):866-72.
- Pozdnyakov A, Kulanthaivelu R, Bauman G, et al. The impact of PSMA PET on the treatment and outcomes of men with biochemical recurrence of prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2022.
Active Surveillance in Prostate Cancer: The 2021 NCCN Controversy
Active Surveillance Background
Prostate cancer represents a public health dilemma: while prostate cancer is the second leading cause of death among men in the US1 and third in Canada,2 it is widely over-diagnosed and over-treated, leading to significant patient anxiety and morbidity.3 Much of the over-diagnosis of prostate cancer relates to the use of serum prostate-specific antigen (PSA) testing for prostate cancer screening, beginning in 1987.4
- Written by: Christopher J.D. Wallis, MD, PhD & Zachary Klaassen, MD, MSc
- References:
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: a cancer journal for clinicians. 2019;69(1):7-34.
- Advisory CCSs, Statistics. CoC. Canadian Cancer Statistics 2014. Toronto, ON: Canadian Cancer Society; 2014.
- Force USPST, Grossman DC, Curry SJ, et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA : the journal of the American Medical Association. 2018;319(18):1901-1913.
- Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. The New England journal of medicine. 1987;317(15):909-916.
- Etzioni R, Gulati R, Falcon S, Penson DF. Impact of PSA screening on the incidence of advanced stage prostate cancer in the United States: a surveillance modeling approach. Med Decis Making. 2008;28(3):323-331.
- Barocas DA, Mallin K, Graves AJ, et al. Effect of the USPSTF Grade D Recommendation against Screening for Prostate Cancer on Incident Prostate Cancer Diagnoses in the United States. The Journal of urology. 2015;194(6):1587-1593.
- Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control. 2008;19(2):175-181.
- Choo R, Klotz L, Danjoux C, et al. Feasibility study: watchful waiting for localized low to intermediate grade prostate carcinoma with selective delayed intervention based on prostate specific antigen, histological and/or clinical progression. The Journal of urology. 2002;167(4):1664-1669.
- Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(3):272-277.
- Musunuru HB, Yamamoto T, Klotz L, et al. Active Surveillance for Intermediate Risk Prostate Cancer: Survival Outcomes in the Sunnybrook Experience. The Journal of urology. 2016;196(6):1651-1658.
- Komisarenko M, Martin LJ, Finelli A. Active surveillance review: contemporary selection criteria, follow-up, compliance and outcomes. Transl Androl Urol. 2018;7(2):243-255.
- Lange JM, Gulati R, Leonardson AS, et al. Estimating and Comparing Cancer Progression Risks under Varying Surveillance Protocols. Ann Appl Stat. 2018;12(3):1773-1795.
- Morash C, Tey R, Agbassi C, et al. Active surveillance for the management of localized prostate cancer: Guideline recommendations. Toronto, ON: Cancer Care Ontario: 2015 Program in Evidence-based Care Guideline No.: 17-9.
- Chen RC, Rumble RB, Loblaw DA, et al. Active Surveillance for the Management of Localized Prostate Cancer (Cancer Care Ontario Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(18):2182-2190.
- Cooperberg MR, Carroll PR. Trends in Management for Patients With Localized Prostate Cancer, 1990-2013. JAMA : the journal of the American Medical Association. 2015;314(1):80-82.
- Loeb S, Byrne N, Makarov DV, Lepor H, Walter D. Use of Conservative Management for Low-Risk Prostate Cancer in the Veterans Affairs Integrated Health Care System From 2005-2015. JAMA : the journal of the American Medical Association. 2018;319(21):2231-2233.
- Briganti A, Fossati N, Catto JWF, et al. Active Surveillance for Low-risk Prostate Cancer: The European Association of Urology Position in 2018. European urology. 2018;74(3):357-368.


