ESMO 2024 Quick Take Insights: A Focus on SPLASH
The ESMO 2024 annual meeting in Barcelona, Spain, featured many practice-changing trials and hypothesis-generating data in genitourinary cancer. One of the most highly anticipated trials in the metastatic castration-resistant prostate cancer (mCRPC) disease space was the phase III SPLASH trial – the focus of this ESMO 2024 Quick Take Insights.
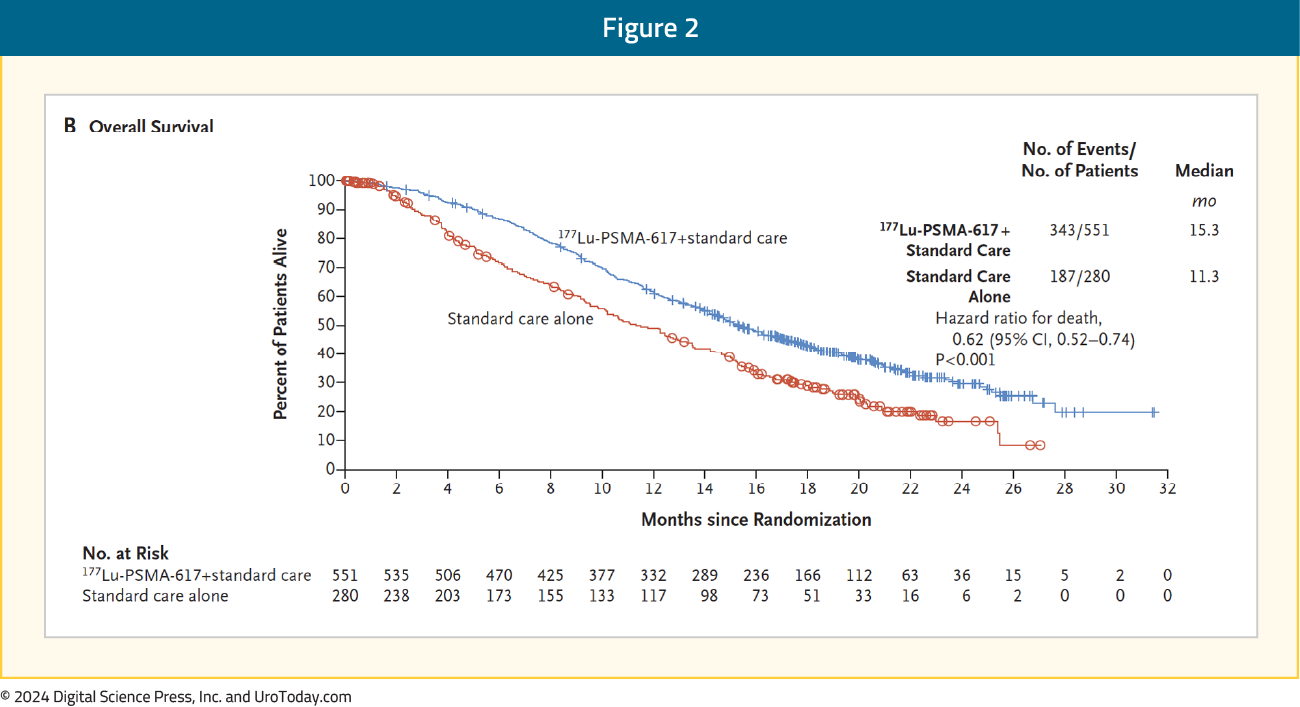
Why is this trial important?Previously, the VISION trial1 led to the approval of 177Lu-PSMA-617 post-docetaxel in the mCRPC setting based on a radiographic progression-free survival (rPFS; HR 0.40, 95% CI 0.29–0.57) and overall survival (OS; HR 0.62, 95% CI 0.52-0.74) benefit when added to standard of care in patients with PSMA-positive disease: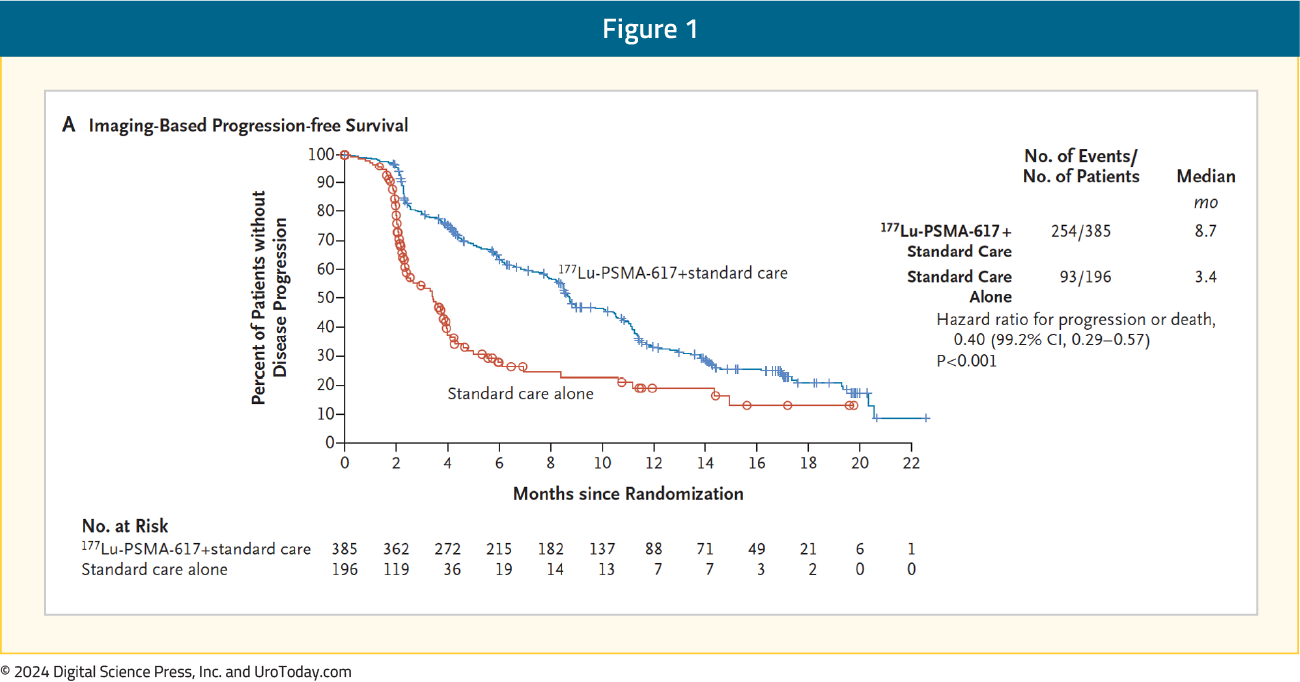
Moreover, PSMAfore2 showed that 177Lu-PSMA-617 (median 11.6 months) demonstrated an rPFS benefit versus androgen receptor pathway inhibitor (ARPI) change (median 5.59 months; HR 0.49, 95% CI 0.39–0.61) in chemotherapy-naïve mCRPC patients, but has not yet been approved in this setting: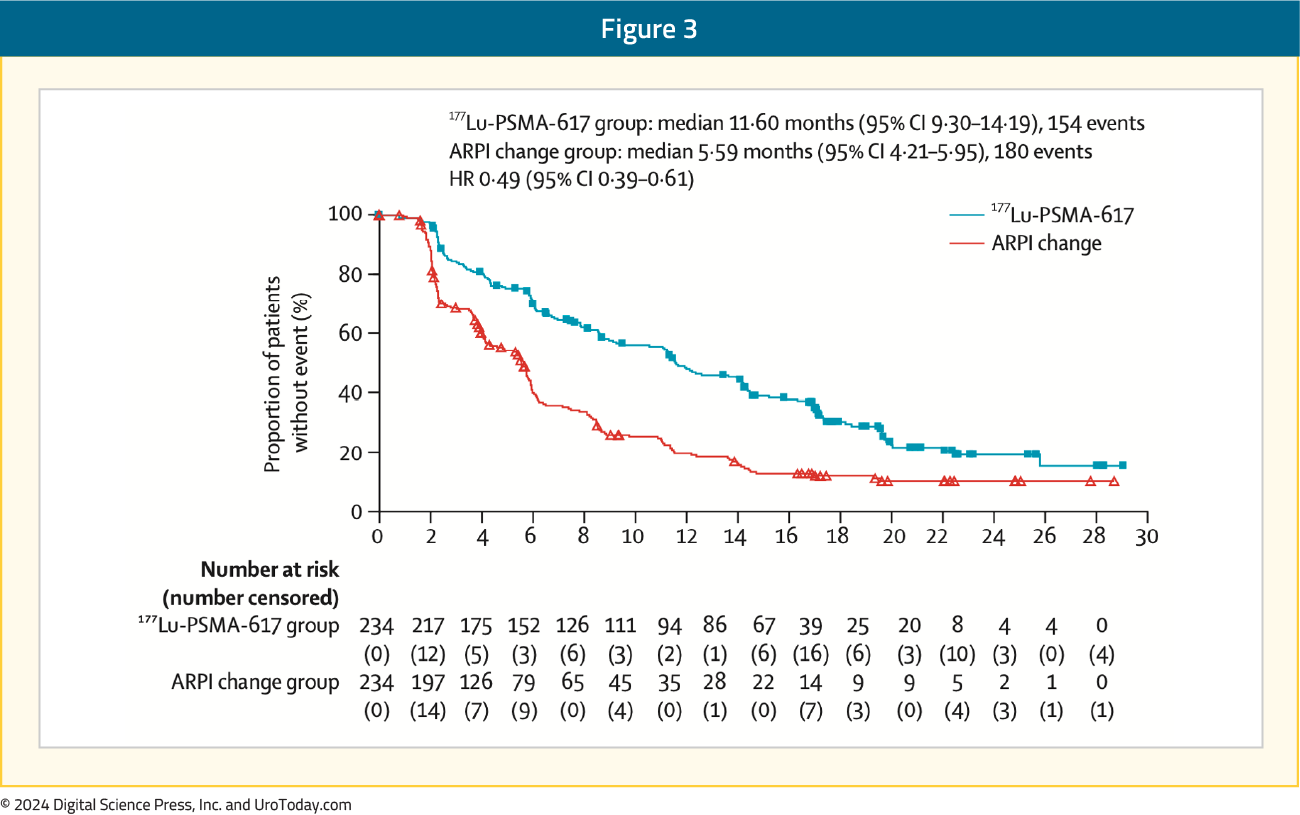
As such, additional radioligand agents in this disease space are needed.
SPLASH is a phase III, randomized trial evaluating 177Lu-PNT2002 in mCRPC patients experiencing disease progression following an ARPI. Patients were randomized 2:1 to 177Lu-PNT2002 every 8 weeks for 4 cycles versus the alternative ARPI (enzalutamide or abiraterone). Patients also had to have PSMA-avid PET imaging and an ECOG performance status of 0-1. The primary endpoint was rPFS and key secondary endpoints included OS and safety: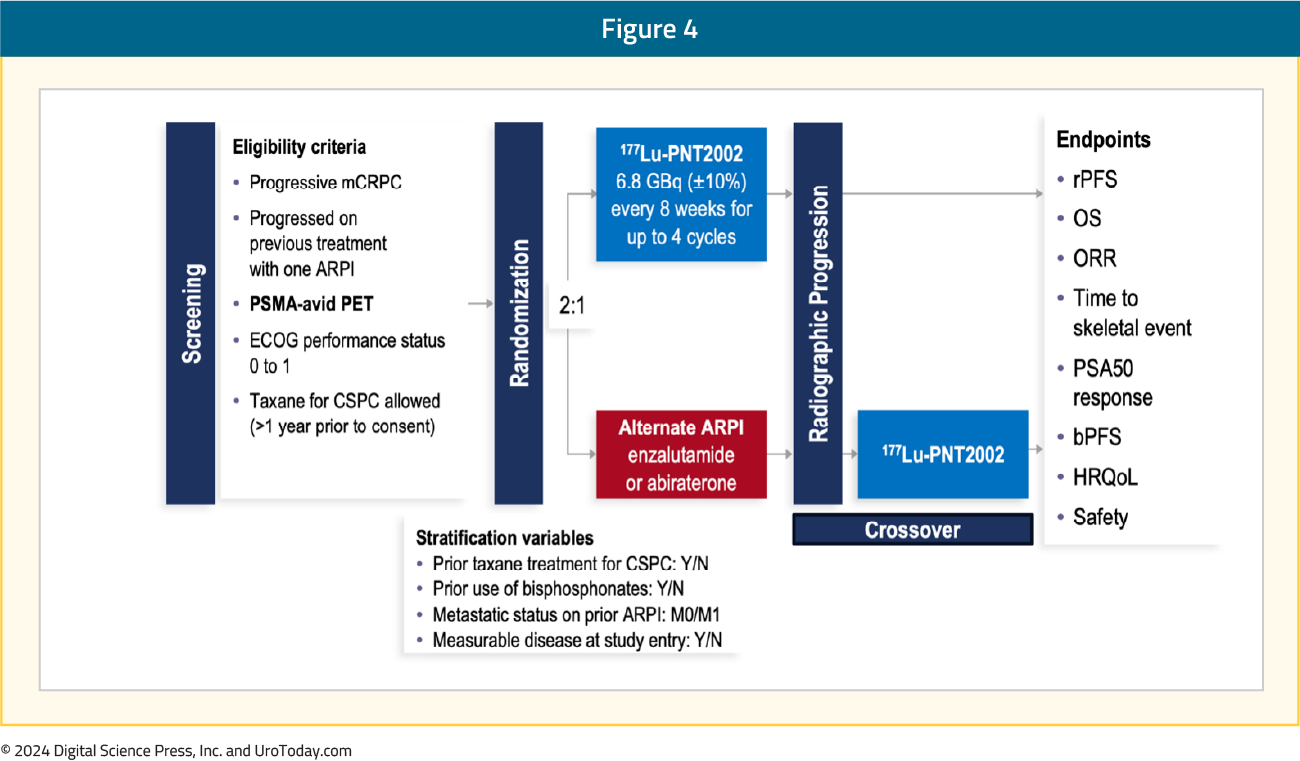
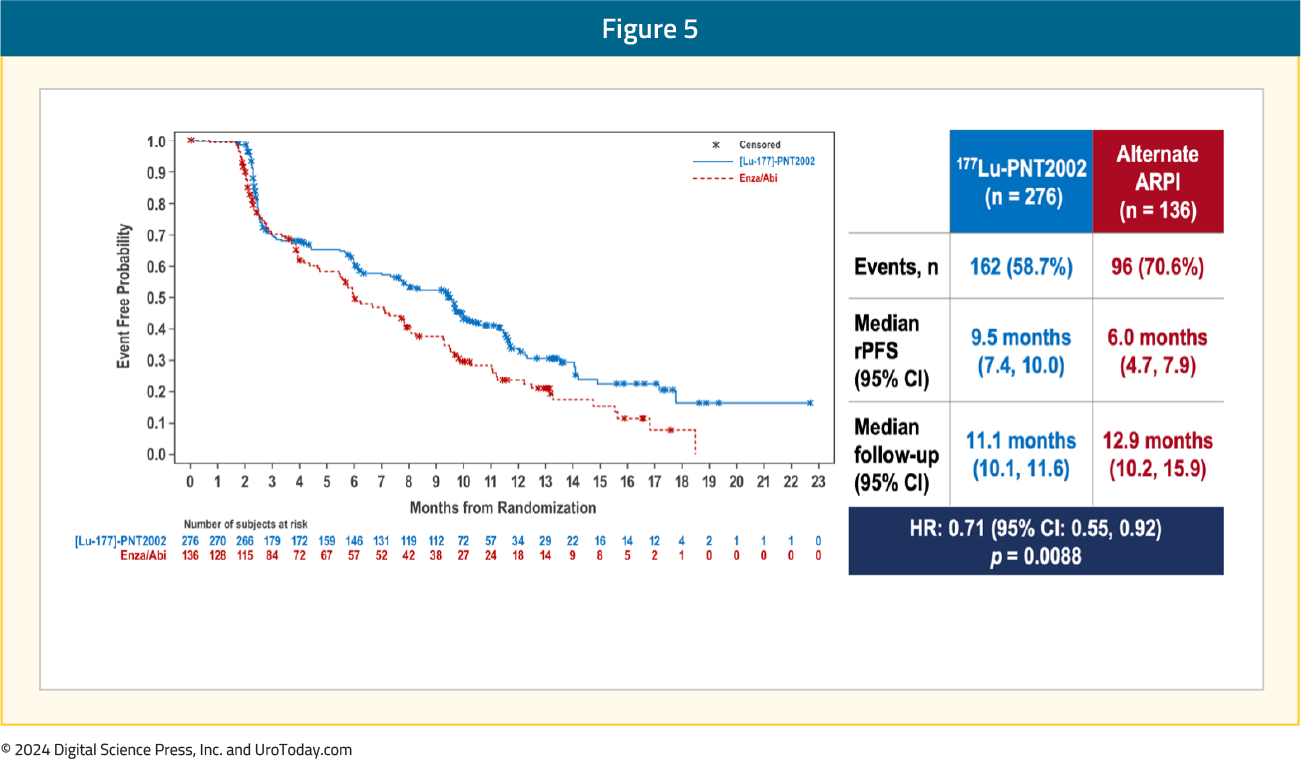
Over a median follow-up of ~12 months, the SPLASH trial met its primary endpoint of an rPFS benefit with 177Lu-PNT2002 (median: 9.5 versus 6 months; HR 0.71, 95% CI 0.55–0.92):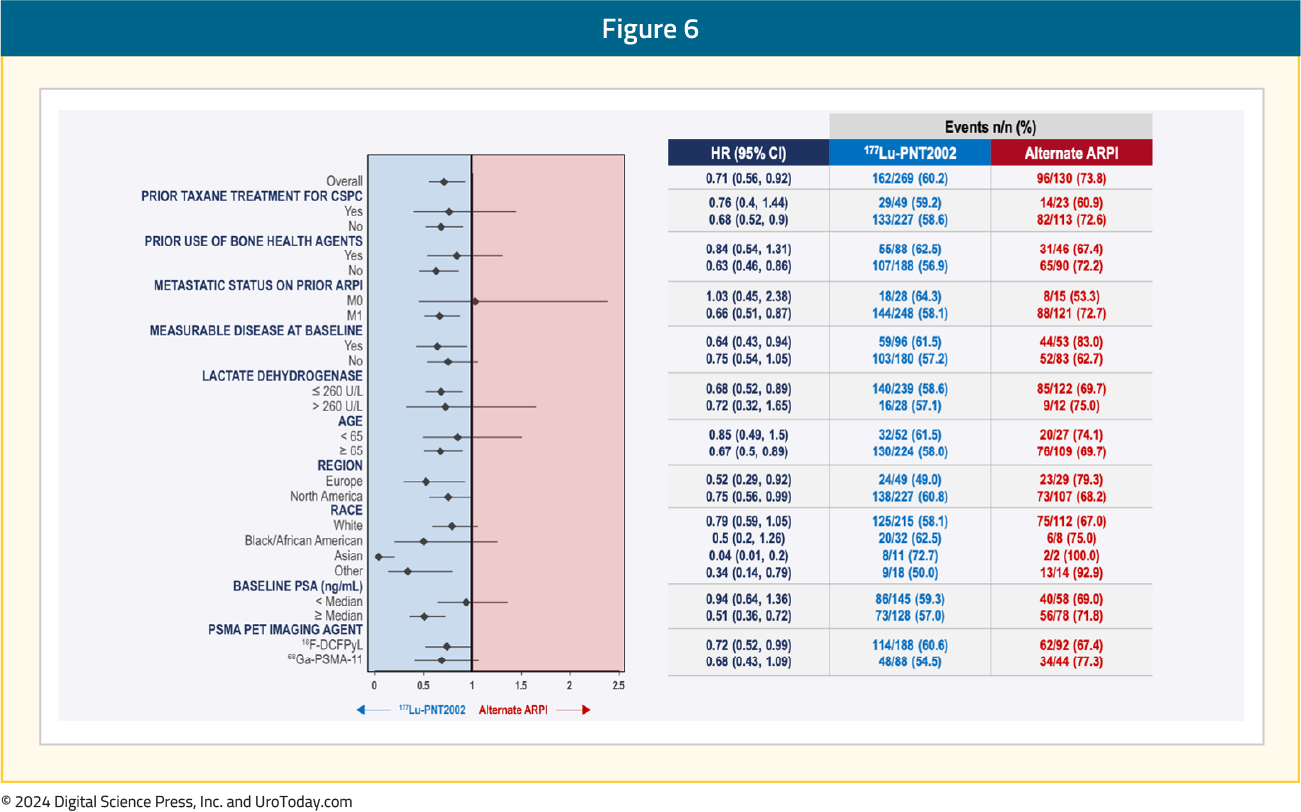
Subgroup analyses demonstrated consistent rPFS benefits with 177Lu-PNT2002 across the evaluated characteristics:
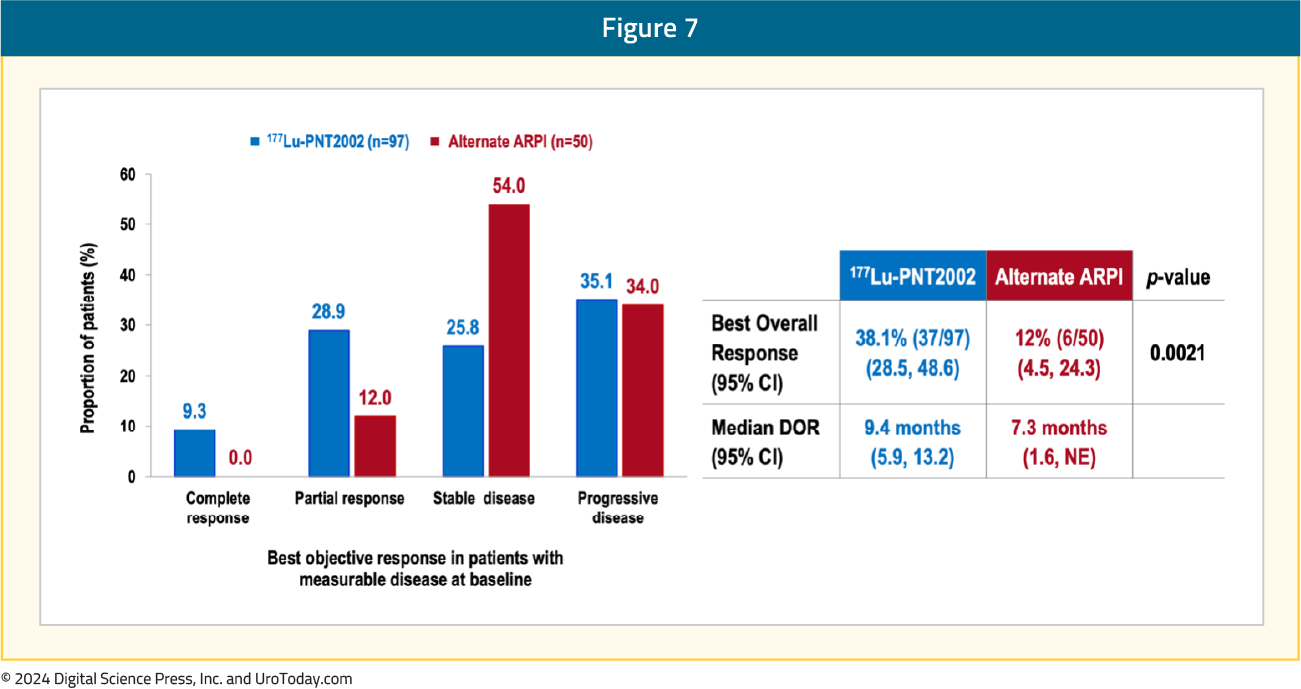
A complete response was observed in 9.3% of patients in the 177Lu-PNT2002 arm, compared to none in the control arm. The best overall response was observed in 38% and 12% of patients, respectively (p=0.0021), and the median durations of response were 9.4 and 7.3 months, respectively:
The first interim analysis of OS did not demonstrate a benefit for 177Lu-PNT2002 (median: 20.8 months versus NE; HR 1.11, 95% CI 0.73–1.69), likely secondary to significant cross-over in the alternate ARPI arm: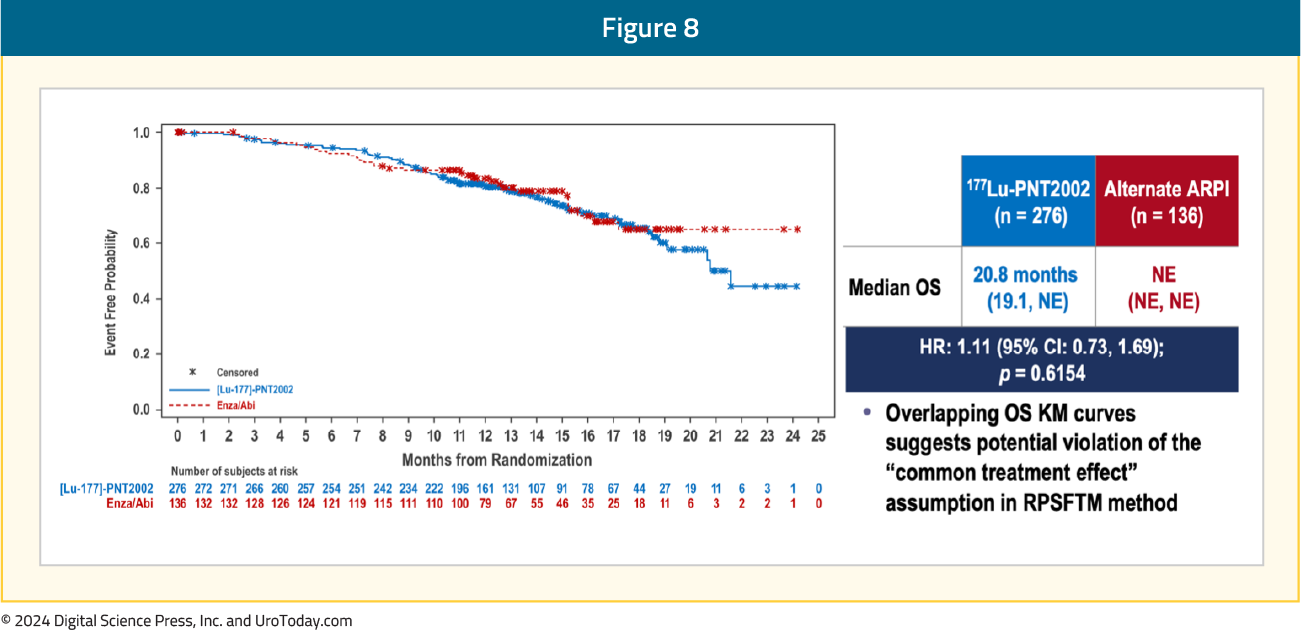
The adverse event profile favored 177Lu-PNT2002 – grade ≥3 events were observed in 10% and 12% of patients in the experimental and control arms, respectively. There were no TEAEs leading to death, and TEAEs leading to treatment discontinuation were observed in 2% and 6% of patients, respectively: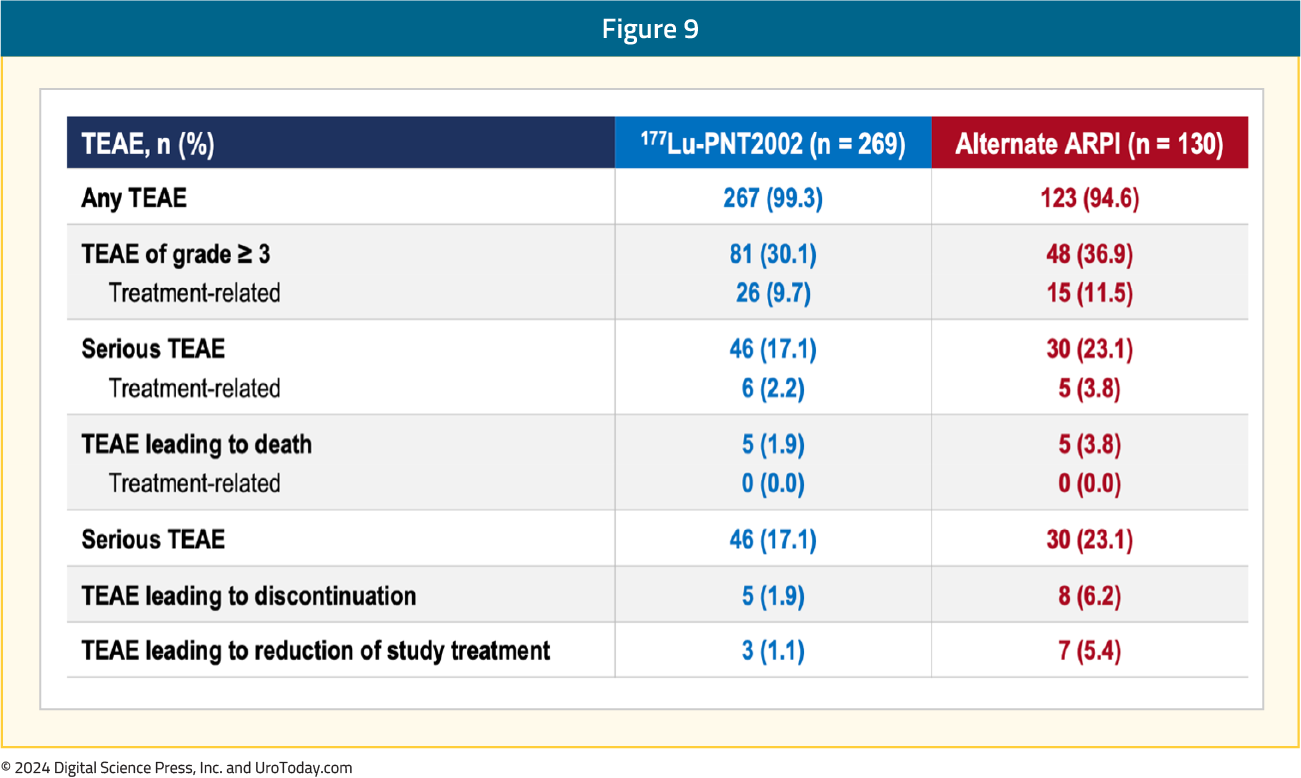
What are the clinical implications?
The SPLASH trial demonstrated that 177Lu-PNT2002 may provide a second radioligand therapy option for patients in the pre-chemotherapy (after an ARPI) mCRPC disease space, with only 4 cycles of treatment. When compared to PSMAfore, the lower dose and longer interval of 177Lu-PNT2002 seems to reduce the rPFS response (9.5 months in SPLASH versus 12 months in PSMAfore), with only a modest reduction in all grade incidence of dry mouth (37.2% in SPLASH vs 57.3% in PSMAfore). With an 84% crossover among the control arms in both trials, OS data may not reach significance, thus the regulatory agencies will likely decide on approval in the pre-chemotherapy mCRPC disease space based on rPFS benefit alone.
Published October 2024


