Introduction
While external beam radiotherapy is a standard treatment option as first-line therapy for men with localized prostate cancer, it is also an important component of care for patients following radical prostatectomy. For approximately two-thirds of patients undergoing radical prostatectomy for prostate cancer, surgery is curative, and patients remain disease-free (without biochemical or radiographic evidence of recurrence).1 However, patients with adverse pathologic findings (defined as seminal vesicle invasion, extraprostatic extension, and positive surgical margins (residual tumor at the surgical site) experience up to a 60% risk of recurrence at 10 years.2There has long been a debate regarding the optimal timing, dosage, and clinical target volume for radiotherapy in patients with either at high risk for or having evidence of biochemical failure following primary definitive treatment in the form of radical prostatectomy. Furthermore, the role of concurrent androgen deprivation therapy, including length of therapy, has long been subject to debate. In this Center of Excellence article, we highlight the recent evidence for radiotherapy in the adjuvant and salvage settings, along with the role of concurrent androgen suppression in this setting.
Timing of radiotherapy: Adjuvant versus Early Salvage
Adjuvant and salvage radiotherapy post-prostatectomy have been defined differently by multiple organizations, including the AUA, ASTRO and NCCN. Currently the NCCN defines post-prostatectomy radiotherapy as follows:
- Adjuvant radiotherapy – (i) typically given within 1-year post-prostatectomy after side effects have stabilized/improved, (ii) there no evidence of recurrence at the start of radiotherapy, (iii) treatment is initiated due to pathologic high-risk features
- Salvage radiotherapy – post-prostatectomy PSA > 0.2 ng/mL followed by another higher value or a single PSA > 0.5 ng/mL
Published at the same times, RAVES also evaluated the role of adjuvant versus early salvage radiotherapy. This non-inferiority phase III trial randomized 333 patients who had undergone a radical prostatectomy and had high-risk features (positive surgical margin, extraprostatic extension, or seminal vesicle invasion) who developed biochemical recurrence following a post-operative PSA level of 0.1 ng/ml or less. Patients were randomized 1:1 to either adjuvant radiotherapy (64 Gy in 32 fractions) within six months or early salvage radiotherapy for a PSA of 0.20 ng/ml or higher. No concurrent androgen deprivation therapy was given. The primary endpoint was freedom from biochemical progression. At a median follow-up of 6.1 years, the 5-year freedom from biochemical progression was non-significantly different (adjuvant: 86% versus salvage: 87%; HR: 1.12, 95% CI: 0.65 – 1.90). 50% of patients in the salvage arm actually received radiotherapy. The grade 2 or worse genitourinary toxicity rate was lower in the salvage radiotherapy group (54% versus 70%), with no difference in grade 2 or worse gastrointestinal toxicity rates (10% versus 14%).4
Also published concurrently, the GETUG-17 trial assessed the role of adjuvant versus early salvage radiotherapy in 424 patients within a similar framework to the other trials. Of note, all patients in this trial received androgen suppression for 6 months. Similar to the RAVES trial, the corresponding independent data monitoring committee recommended early termination of enrolment because of unexpectedly low event rates. At a median follow-up of 75 months, 54% of patients in the early salvage arm received radiotherapy. There was no significant difference in the primary study outcome of 5-year event-free survival: 92% (95% CI: 86 – 95) in the adjuvant arm and 90% (95% CI: 85 – 94%) in the early salvage arm (HR: 0.81, 95% CI: 0.48 – 1.36, log-rank p=0.42). Acute grade 3 or worse toxic effects occurred in six (3%) of 212 patients in the adjuvant radiotherapy group and in four (2%) of 212 patients in the salvage radiotherapy group.5
Following the completion of these three aforementioned trials, results from the pre-planned ARTISTIC analysis, which utilized a prospective framework for adaptive meta-analysis, were published. This meta-analysis utilized a harmonized definition of event-free survival, defined as the time from randomization until the first evidence of either biochemical progression (PSA ≥0.4 ng/mL and rising after completion of any postoperative radiotherapy), clinical or radiological progression, initiation of a non-trial treatment, death from prostate cancer, or a PSA level of at least 2.0 ng/mL at any time after randomization. With 1,075 patients included at a median follow-up of 60 to 78 months, this meta-analysis demonstrated no significant improvement in event-free survival with adjuvant radiotherapy compared to early salvage treatment (HR: 0.95, 95% CI: 0.75-1.21, p=0.70), with only a one percentage point (95% CI: 2 - 3) change in 5-year event-free survival (89% vs 88%).6
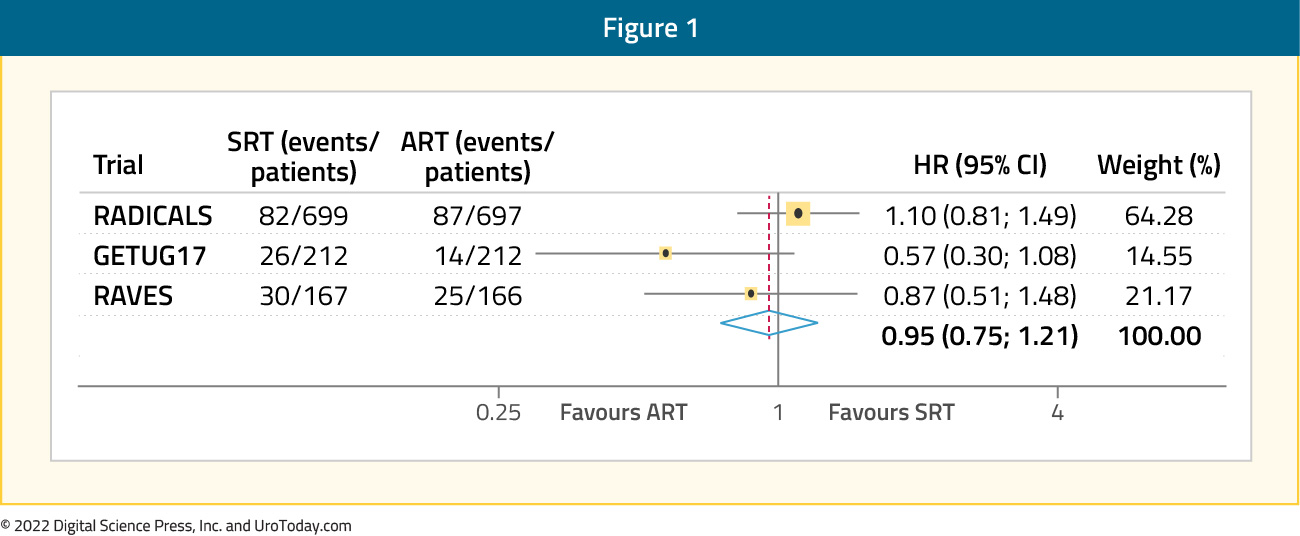
Duration of Concurrent Androgen Deprivation Therapy During Adjuvant/Salvage Radiotherapy
Results of the RADICALS-HD trial were recently presented at ESMO 2022. This arm of the RADICALS platform is a randomized comparison assessing the concurrent use and duration of androgen suppression in patients with an indication for post-operative radiotherapy who had not received prior post-operative androgen deprivation therapy. Notably, those with prior pelvic radiotherapy, prior hormonal therapy, metastatic disease, or a PSA over 5 ng/mL were excluded. Following radical prostatectomy but prior to the initiation of their radiotherapy, patients were randomized to either no ADT (“None”), 6 months ADT (“Short”), or 24 months ADT (“Long”).
The trial was powered for the two pairwise comparisons. The primary outcome measure was metastasis-free survival with secondary outcomes including time to salvage androgen deprivation therapy and overall survival. Between 2007 and 2015, 2,839 patients were enrolled in RADICALS-HD from UK, Canada, and Denmark. The median age of included men was 66 years, 23% had pT3b/T4 disease, 20% had Gleason 8-10 histology, and the median pre-radiotherapy PSA was 0.22ng/ml (consistent with an early salvage treatment intent). Of the 2,389 patients in RADICALS-HD, 492 participated in the 3-way randomization. Including both 2-way and 3-way randomization, 1,480 patients contributed to the None versus Short comparison and 1,523 patients contributed to the Short versus Long comparisons. Over a median follow-up of 9 years, in the None versus Short comparison, based on 268 metastasis-free survival events, 6 months of androgen suppression did not improve metastasis-free survival compared to no androgen deprivation (HR: 0.89; CI: 0.69 - 1.14; 79% vs 80% event-free at 10 years). Similarly, overall survival was not improved (HR: 0.88; 95% CI: 0.65 - 1.19) nor was freedom-from-distant-metastasis (HR: 0.82; CI: 0.58 - 1.15). However, the time to salvage ADT was delayed (HR: 0.54; 95% CI: 0.42 - 0.70):

In the comparison of Short versus Long duration of androgen deprivation therapy, based on 313 metastasis-free survival events, 24 months of ADT improved metastasis-free survival (HR: 0.77; 95% CI: 0.61 - 0.97; 72% vs 78% at 10 years), freedom-from-distant-metastases (HR: 0.63; 95% CI: 0.47 - 0.85), and delayed the time to salvage ADT (HR: 0.73; 95% CI: 0.59 - 0.91). No significant improvements in overall survival were noted (HR: 0.88, 95% CI: 0.66 – 1.17):

Notably, results of the None versus Long comparison did not demonstrate a metastasis-free survival benefit (HR: 0.94, 95% CI: 0.53 – 1.68). Use of androgen suppression was not associated with a significant increase in toxicity as measured using RTOG grades.
Similar to the ARTISTIC trial framework, the DADSPORT (Duration of Androgen Suppression with Post-Operative Radiotherapy) meta-analysis pooled data from four trials evaluating androgen deprivation therapy use with post-operative radiotherapy. This meta-analysis included results from RG/RTOG 9601, GETUG-AFU 16, NRG/RTOG 0534, and RADICALS-HD.

The primary analysis was a fixed-effect, inverse-variance meta-analysis, stratified by hormone duration (no hormone therapy, 6 months, and 24 months of hormone therapy) following radiotherapy. A pre-specified sensitivity analysis excluded men from NRG/RTOG 9601 who had PSA >1.5 ng/ml at randomization. This analysis included a total of 4,452 men with a median follow-up of ≥8 years. There was no clear improvement in overall survival with hormone therapy compared to no hormone therapy (HR: 0.87, 95% CI: 0.75 – 1.01), irrespective of duration of androgen suppression:

Based on data from three trials (653 events, 3364 men; 100%), there was evidence that 6 months of hormone therapy improved metastasis-free survival compared to no hormone therapy (HR: 0.82, 95% CI: 0.70 - 0.96, p = 0.01), with a 5-year absolute improvement of 2% (95% CI: 0% - 3%).The authors concluded that there was no clear evidence of difference in survival with either 6 or 24 months of hormone therapy versus none, and there was no clear evidence of a difference between 6 versus 24 months.

Combination of Prostate and Pelvic Nodal Radiotherapy with Androgen Deprivation Therapy
Results of the much-anticipated NRG Oncology/RTOG 0534 SPPORT trial have recently been published in The Lancet in May 2022. This was an international, multicenter randomized trial of 1,792 patients who previously underwent a radical prostatectomy and have evidence of residual disease (i.e. persistent PSA post-operatively) or a rising PSA of between 0.1 and 2.0 ng/ml. Patients who had previously underwent a lymphadenectomy were eligible, given that they had no clinical or pathologic evidence of nodal involvement. Other eligibility criteria included pT2 or pT3 disease, prostatectomy Gleason Score of 9 or less, and a Zubrod performance status of 0–1. Patients were equally randomized into 1one of three arms:- Group 1: Prostate-bed radiotherapy (64.8 – 70.2 Gy; 1.8 Gy/fraction)
- Group 2: Prostate-bed radiotherapy + short-term ADT (4-6 months)
- Group 3: Prostate-bed radiotherapy + short-term ADT + pelvic nodal radiotherapy
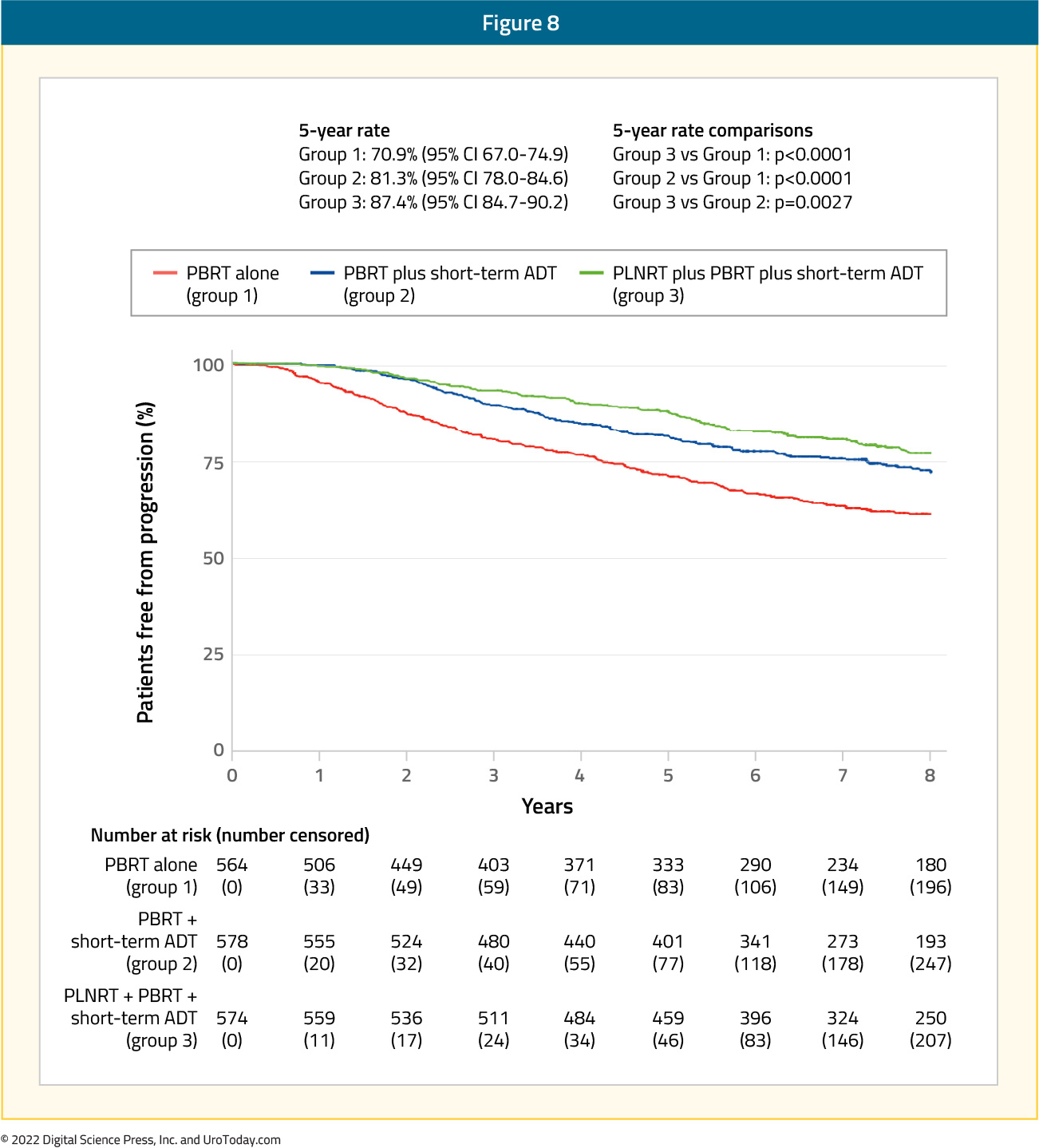
Acute grade 2 or worse adverse events were significantly higher in group 3 (44%), followed by group 2 (36%) and group 1 (18%; p for all < 0.0001). However, late toxicity (>3 months after radiotherapy) did not differ significantly between the groups, except for later grade 2 or worse blood or bone marrow events in group 3 versus group 2 (one-sided p = 0.0060).7 This trial established the importance of concurrent ADT use and pelvic nodal irradiation in the salvage setting.
Androgen Receptor Axis-Targeted Treatment Intensification with Salvage Radiotherapy
Presented at ASCO 2022, the SALV-ENZA trial is a phase II study evaluating salvage radiotherapy with or without enzalutamide for patients with high-risk biochemical recurrence following radical prostatectomy. Patients were randomized 1:1 to salvage radiotherapy plus either enzalutamide 160 mg daily or placebo. Study drug therapy was given for 6 months with salvage radiotherapy to the prostate only (66.6 – 70.2 Gy) given following 2 months of therapy. The primary endpoint was freedom-from-PSA progression. This trial included 86 patients, with a median pre-salvage treatment PSA of 0.3 ng/ml, 56/86 (65%) had extra-prostatic disease (pT3), 39/86 (45%) had Gleason Grade Group 4 or higher, and 43/96 (50%) had positive surgical margins. Over a median follow-up of 34 (range 0-52) months, freedom-from-PSA progression was significantly improved with enzalutamide (compared to placebo) with a hazard ratio of 0.40 (95% CI: 0.17 - 0.92, p = 0.027):
At the 2-year landmark, freedom-from-PSA progression was 87.1% vs 68.1%, respectively. Subgroup analyses demonstrate evidence of effect modification (p-value of interaction = 0.019) with a differential benefit of enzalutamide in men with pT3 (HR: 0.22, 95% CI: 0.07- 0.69) versus pT2 disease (HR: 1.54, 95% CI: 0.43 - 5.47). There was also evidence of a greater benefit (p-value of interaction = 0.023) among those with positive surgical margins (HR: 0.14, 95% CI: 0.03 - 0.64) compared to those with negative margins (HR: 1.00, 95% CI: 0.36 - 2.76). The most common adverse events were grade 1-2 fatigue (13% enzalutamide vs 9%) and urinary frequency (6 % enzalutamide vs 8%).

Further trials in this disease space will evaluate the role of ARAT addition to radiotherapy in the adjuvant/salvage setting. NRG GU-006 (NCT03371719) is a phase II biomarker enhanced anti-androgen trial for intermediate risk prostate cancer, utilizing biomarker stratified PAM 50. Men will be enrolled who have a PSA of 0.1-1.0 ng/mL (or persistent PSA after radical prostatectomy 0.04-0.2 ng/mL). Randomization will be to radiation alone versus radiation + apalutamide.
The FORMULA-509 trial (NCT03141671) will assess enhanced androgen ablation and abiraterone + prednisone in intermediate/high risk patients as follows:

The RTOG 3506 (STEEL) trial (NCT03809000) will assess standard versus enhanced ADT (for 24 months) in patients with high-risk prostate cancer:

Genomic Classifiers Risk Stratification in Salvage Radiotherapy Setting
The results of two ancillary studies evaluating Decipher® genomic analysis of radical prostatectomy specimens of patients receiving salvage radiotherapy have recently been published. In 2021, results of an ad-hoc analysis of the NRG/RTOG 9601 phase III trial that included patient undergoing salvage radiotherapy with or without 2 years of bicalutamide were published. This ancillary study used radical prostatectomy specimens from the trial conducted between March 1998 and March 2003. The specimens were centrally reviewed, and RNA was extracted from the highest-grade tumor available. Clinical-grade whole transcriptomes from samples passing quality control were assigned genomic classifier scores (scale of 0 to 1). The primary objective was to validate the independent prognostic ability of this genomic classifier for distant metastases, with secondary endpoints of prostate cancer-specific and overall mortality. Utilizing samples from 352 men with a median follow-up of 13 years, the authors demonstrated that the Decipher® genomic classifier, modeled as a continuous variable (per 0.1 unit), was independently associated with rates of distant metastases (HR: 1.17, 95% CI: 1.05 - 1.32, p = 0.006), prostate cancer-specific mortality (HR: 1.39, 95% CI: 1.20 - 1.63, p < 0.001), and overall survival (HR: 1.17, 95% CI: 1.06 -1.29, p = 0.002), after adjusting for age, race/ethnicity, Gleason score, T stage, margin status, entry PSA, and treatment arm. The estimated absolute effect of bicalutamide on 12-year overall survival was less when comparing patients with lower versus higher genomic classifier scores (2.4% vs 8.9%), which was further demonstrated in men receiving early salvage radiotherapy at a PSA antigen level lower than 0.7 ng/mL (-7.8% vs 4.6%). Results of this study suggest that not all men with biochemical recurrence following surgery benefit equally from addition of hormone therapy to salvage radiotherapy, and that perhaps treatment intensification with androgen suppression should be reserved for those with higher Decipher® genomic classifier scores.8
A similar post-hoc analysis of the SAKK 09/10 trial (Swiss Group for Clinical Cancer Research) was conducted by Dal Pra et al. This trial included 350 men with biochemical recurrence after radical prostatectomy who received salvage radiotherapy (64 – 70 Gy) without concurrent hormonal therapy or pelvic nodal radiotherapy. The primary endpoint was biochemical progression, whereas secondary endpoints were clinical progression and time to hormone therapy. The analytic cohort included 226 patients, with a median follow-up of 6.3 years. The Decipher® genomic classifier score (high versus low-intermediate) was independently associated with the rates of biochemical progression (sHR: 2.26, 95% CI: 1.42 - 3.60; p < 0.001), clinical progression (HR: 2.29, 95% CI: 1.32 - 3.98; p = 0.003), and use of hormone therapy (sHR: 2.99, 95% CI: 1.55 - 5.76; p = 0.001). Genomic classifier high patients had a 5-year freedom from biochemical progression of 45% versus 71% for genomic classifier low-intermediate. Dose escalation did not benefit the overall cohort or either genomic classifier subgroup. Similar to the aforementioned study by Feng et al., the authors concluded that results of their analysis suggest that the Decipher® genomic classifier score can be used to personalize the concurrent use of systemic therapy in the post-operative salvage setting.9
Novel Imaging Techniques to Guide Salvage Radiotherapy
With the increased utilization of molecular imaging (ie with 18F-fluciclovine and prostate-specific membrane antigen (PSMA) PET/CT) to guide treatment decision making and planning in the biochemical recurrent disease space, the EMPIRE-1 trial sought to evaluate whether 18F-fluciclovine improves cancer control compared to conventional imaging (bone scan + CT/MRI) alone for salvage post-prostatectomy radiotherapy. This was a single center, open label, phase 2/3 trial of post-prostatectomy patients with a detectable post-operative PSA and negative conventional imaging. These patients were randomly assigned in a 1:1 ratio to radiotherapy directed by conventional imaging alone or to conventional imaging plus 18F-fluciclovine-PET/CT. In the 18F-fluciclovine-PET/CT group, radiotherapy decisions were rigidly determined by PET findings, which were also used for target delineation. The primary study outcome was 3-year event-free survival, with events defined as biochemical or clinical recurrence or progression, or initiation of systemic therapy. Between 2012 and 2019, this trial recruited 165 patients, with a median follow-up of 3.52 years. The 3-year event-free survival was significantly improved in the 18F-fluciclovine arm: 75.5% versus 63.0% (difference 12.5; 95% CI: 4.3–20.8; p=0·0028). This was confirmed on adjust analysis (HR: 2.04, 95% CI: 1.06 – 3.93, p = 0.0327). The toxicity profile tended to favor those in the 18F-fluciclovine arm, although results were non-significantly different.10Conclusions
Recent randomized evidence strongly supports early salvage radiotherapy, rather than adjuvant radiotherapy, for patients at high risk of biochemical recurrence following radical prostatectomy. The role of concurrent ADT with postoperative radiotherapy remains somewhat controversial though emerging data suggests a role of molecular biomarkers to better delineate populations of patients who will derive the greatest benefit. Moving forward, ongoing work will better define the role of further treatment intensification and of molecular imaging in this disease space.Written by:
- Rashid Sayyid, MD MSc, University of Toronto, Toronto, ON
- Zachary Klaassen, MD MSc, Medical College of Georgia, Augusta, Georgia, USA


