External beam radiotherapy, along with radical prostatectomy, has been a mainstay treatment option for prostate cancer for decades and is currently recommended by numerous guidelines for the treatment of intermediate- and high-risk disease.1-3 While it is clear that radiotherapy should include the tumor, the prostate, and seminal vesicles, the role of prophylactic pelvic nodal irradiation for patients without overt evidence of regional pelvic nodal involvement has long been debated.
This center of excellence article reviews the current evidence assessing the utility of pelvic nodal irradiation and focal boost to the intraprostatic tumor.
Pelvic Nodal Irradiation
The rationale for pelvic nodal irradiation is to eradicate nodal micrometastases, providing better regional control and improved survival outcomes. Although newer imaging modalities in the form of positron emission tomography (PET)-based techniques provide improved sensitivity for detection of regional lymph node spread, this modality remains unreliable for the detection of small (<5 mm deposits).4 Currently, the risk of pelvic nodal involvement is estimated using the Roach formula, which incorporates serum PSA levels at diagnosis and tumor Gleason Score,5 and it has been proposed that patients with a Roach nodal risk of 20 to 40% would be those most likely to benefit from prophylactic pelvic nodal irradiation. It bears mention that most patients with sufficient oncologic risk from their prostate cancer as to warrant prophylactic pelvic nodal irradiation will have indications for concurrent androgen deprivation therapy. To date, three large, randomized trials have sought to assess the role of prophylactic pelvic nodal irradiation in clinically localized prostate cancer.
The NRG/RTOG 9413 trial was a 2 x 2 factorial study that evaluated the role of whole pelvic radiotherapy (versus prostate only) and timing of hormone therapy (neoadjuvant versus adjuvant). This phase III trial randomized 1,322 patients with prostate cancer who had an estimated risk of lymph node involvement of at least 15% in a 1:1:1:1 fashion to one of four treatment arms: whole pelvic radiotherapy plus neoadjuvant hormone suppression, prostate only radiotherapy plus neoadjuvant hormone suppression, whole pelvic radiotherapy plus adjuvant hormone suppression, and prostate only radiotherapy plus adjuvant hormone suppression. Neoadjuvant ADT was initiated 2 months before radiotherapy and was continued until radiotherapy completion, whereas adjuvant androgen deprivation therapy was given at the completion of radiotherapy for 4 months. The pelvic radiation dose was 70 Gy. Updated 10-year results were published in Lancet Oncology in 2018 with a median follow-up of 8.8 years. Progression-free survival across all time points differed significantly across the four treatment groups (p = 0.002). The 10-year progression free survival estimate was 28.4% (95% CI: 23.3 – 33.6%) in the whole pelvic irradiation + neoadjuvant hormone arm compared to 23.5% (95% CI: 18.7 – 28.3%) for the prostate-only radiotherapy + neoadjuvant arm, 19.4% (95% CI: 14.9 – 24.0%) in the whole pelvic irradiation + adjuvant arm, and 30.2% (95% CI: 25.0 – 35.4%) in the prostate-only radiotherapy + adjuvant arm:
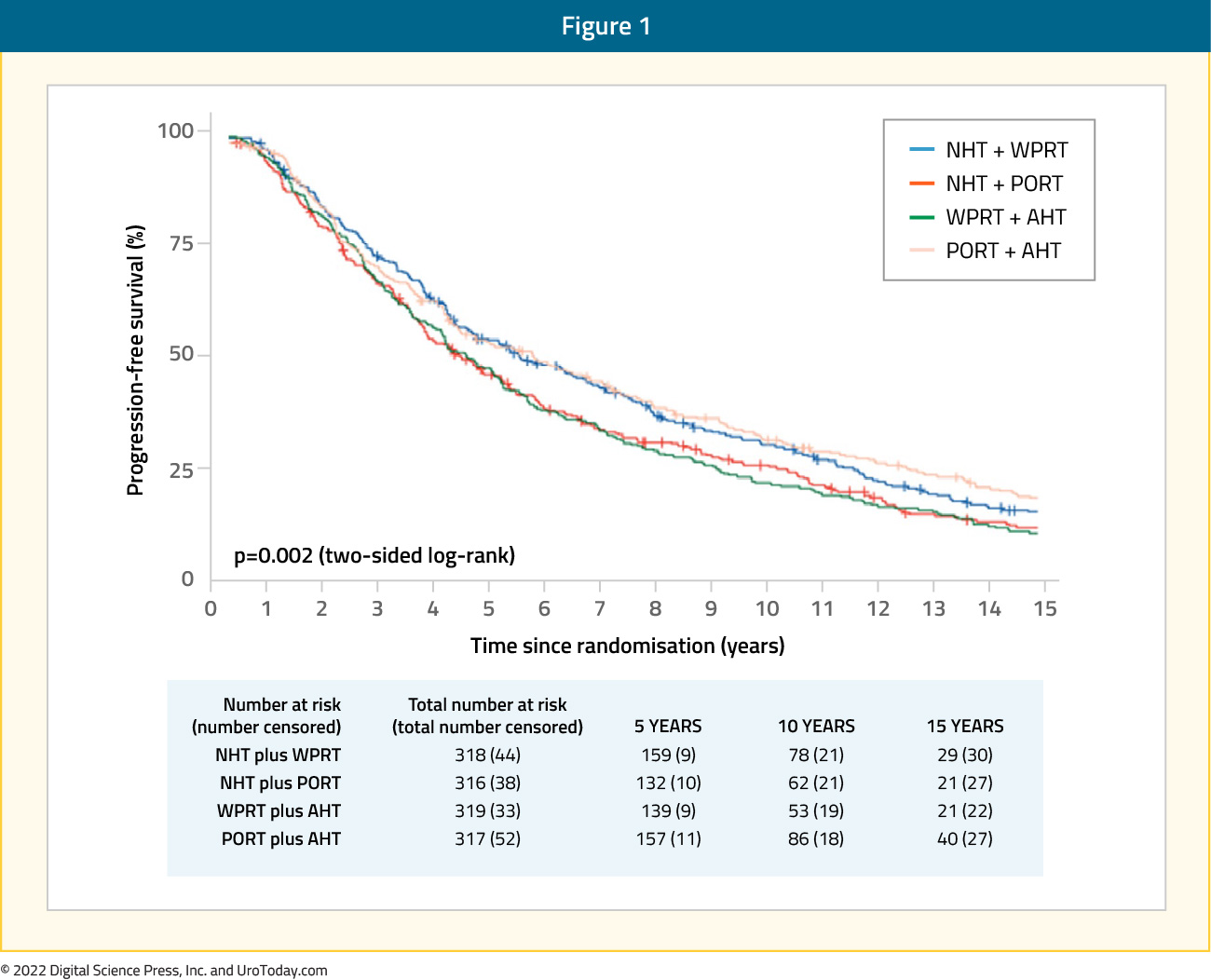
There were no significant differences in grade 3 or worse late bladder toxicities between the four arms, however late grade 3 or worse gastrointestinal adverse events occurred in 7% of patients in the whole pelvis + neoadjuvant arm compared to 2-3% in the other arms.6
The GETUG-01 trial randomized 446 patients with T1b-T3N0pNxM0 prostate cancer to either prostate-only or prostate plus pelvic nodal irradiation. Patients were stratified into two groups: "low risk" (cT1-T2 and Gleason Score 6 and PSA <3× the upper normal lab limit, n=92) versus "high risk" (cT3, Gleason Score >6, or PSA >3× the upper normal lab limit). High-risk patients received 6 months of neoadjuvant and concomitant hormonal therapy. The total doses administered were 66 to 70 Gy to the prostate and 46 Gy to the pelvic nodes. After a median follow-up of 11.4 years, the 10-year overall and event-free survival rates were similar in the two treatment arms. Slightly improved event-free survival was noted in the low-risk subgroup in favor of pelvic nodal radiation therapy (77.2% vs 62.5%; p =0.18). A post hoc subgroup analysis showed a significant benefit of pelvic irradiation when the risk of lymph node involvement was <15% (Roach formula). This benefit seemed to be limited to patients who did not receive hormonal therapy.7
Finally, results of the POP-RT trial were published in 2021 in the Journal of Clinical Oncology. This phase III trial randomized 224 prostate cancer patients with clinically node negative disease and an estimated nodal risk ≥ 20% to either prostate-only radiation therapy (68 Gy in 25 fractions) or whole pelvic radiotherapy (68 Gy in 25 fractions to the prostate plus 50 Gy to the pelvic nodes, including common iliac). All patients received image-guided, intensity-modulated radiotherapy and a minimum of two years of androgen deprivation therapy. The primary end point was 5-year biochemical failure-free survival. Over a median follow-up of 68 months, the 5-year biochemical failure-free survival was superior in the whole pelvis arm (95% versus 81.2%; HR: 0.23; p < 0.001) as were 5-year disease-free (89.5% versus 77.2%; HR: 0.40, p = 0.002) and distant metastasis-free survivals (95.9% versus 89.2%; HR: 0.35, p = 0.01). No significant differences in 5-year overall survival rates were noted (92.5% versus 90.8%, HR: 0.92, p = 0.93):8
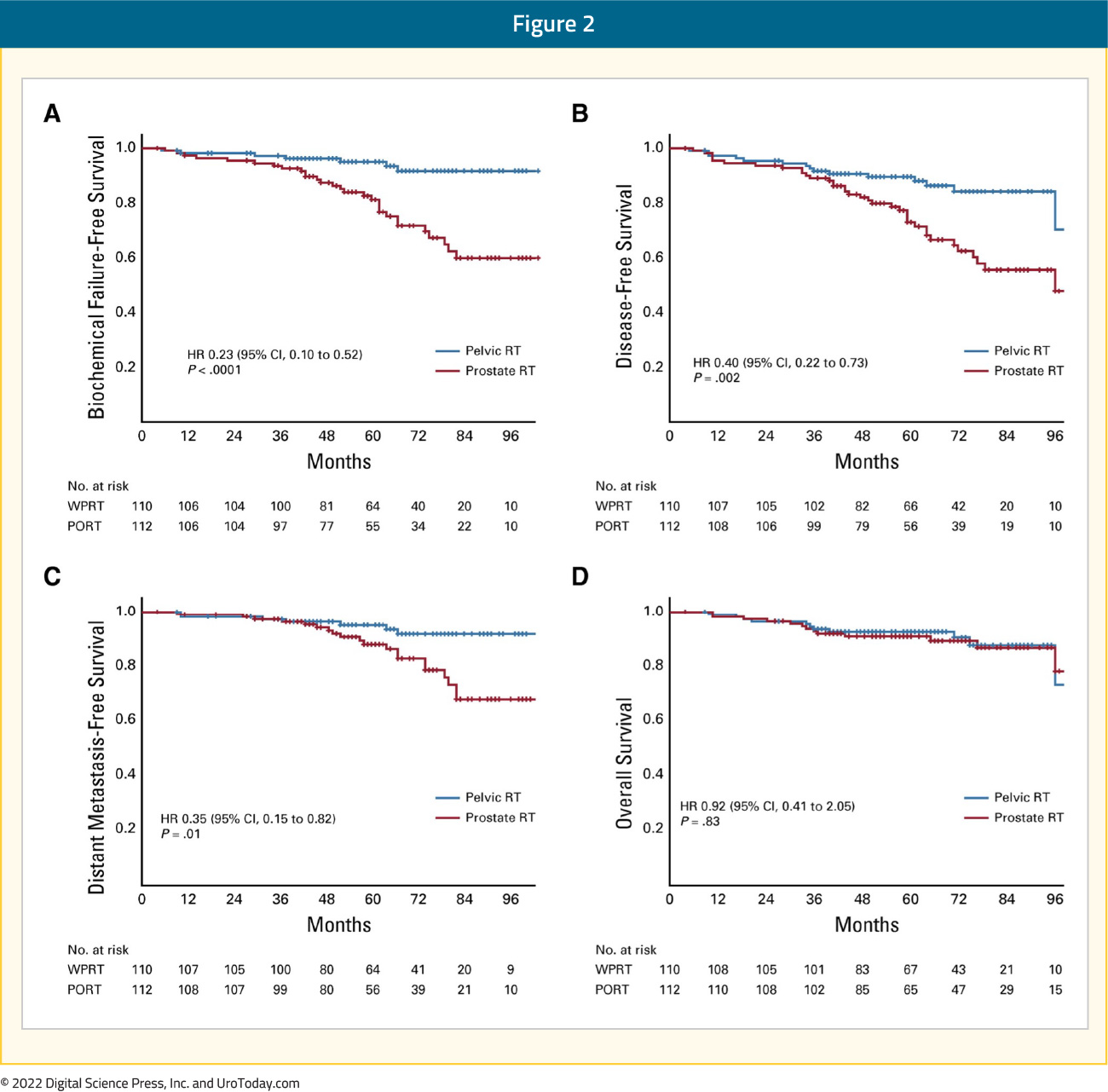
There were several notable differences between the POP-RT and the RTOG 9413/GETUG-01 trials. Patients included in the POP-RT trial were very high-risk patients with a median PSA of 28.2 ng/ml, Gleason Group 4-5 disease for almost half of the patients, and a median estimated Roach nodal risk of 37.8%. All patients in the POP-RT trial received androgen suppression for 24 months compared to either no ADT or 4-8 months in the GETUG-01 and RTOG 9413 trials, respectively. Furthermore, the superior border of the pelvic field in POP-RT was higher at L4-L5 and included the common iliac nodes, whereas GETUG-01 was set at S1-S2 and RTOG 9413 at L5-S1. Most importantly though, approximately 80% of patients in the POP-RT trial were staged pre-treatment with a PET/CT, thus excluding patients with higher volume of nodal or distant metastatic disease that may have been missed on conventional imaging. These differences collectively suggest that the benefit of adding pelvic nodal radiotherapy is likely most pronounced in patients without distant micrometastases on PSMA PET/CT, receiving adequate androgen suppression, and who are at significant risk for locoregional micrometastases.
Glicksman et al. recently reported on the long-term results of pelvic nodal irradiation with a simultaneous hypofractionated integrated prostate boost for localized high risk prostate cancer patients. All patients had evidence of high-risk features (cT3, PSA of 20 – 100 ng/ml, and/or Gleason Score 8-10) and no evidence of nodal or distant metastasis on an abdominopelvic CT or bone scan. Patients received 45 Gy in 25 fractions to the prostate and pelvic lymph nodes with a simultaneous intensity-modulated radiotherapy boost of 22.5 Gy to the prostate (total dose of 67.5 Gy in 25 fractions), with androgen deprivation therapy for two to three years. The primary study endpoint was biochemical failure, with secondary endpoints of distant metastases and overall survival. This phase II trial included 230 patients with a median follow up of 11.2 years. At 10 years, the cumulative incidence of biochemical failure was 33.4%, distant metastasis was 16.5%, and overall survival was 76.3%:
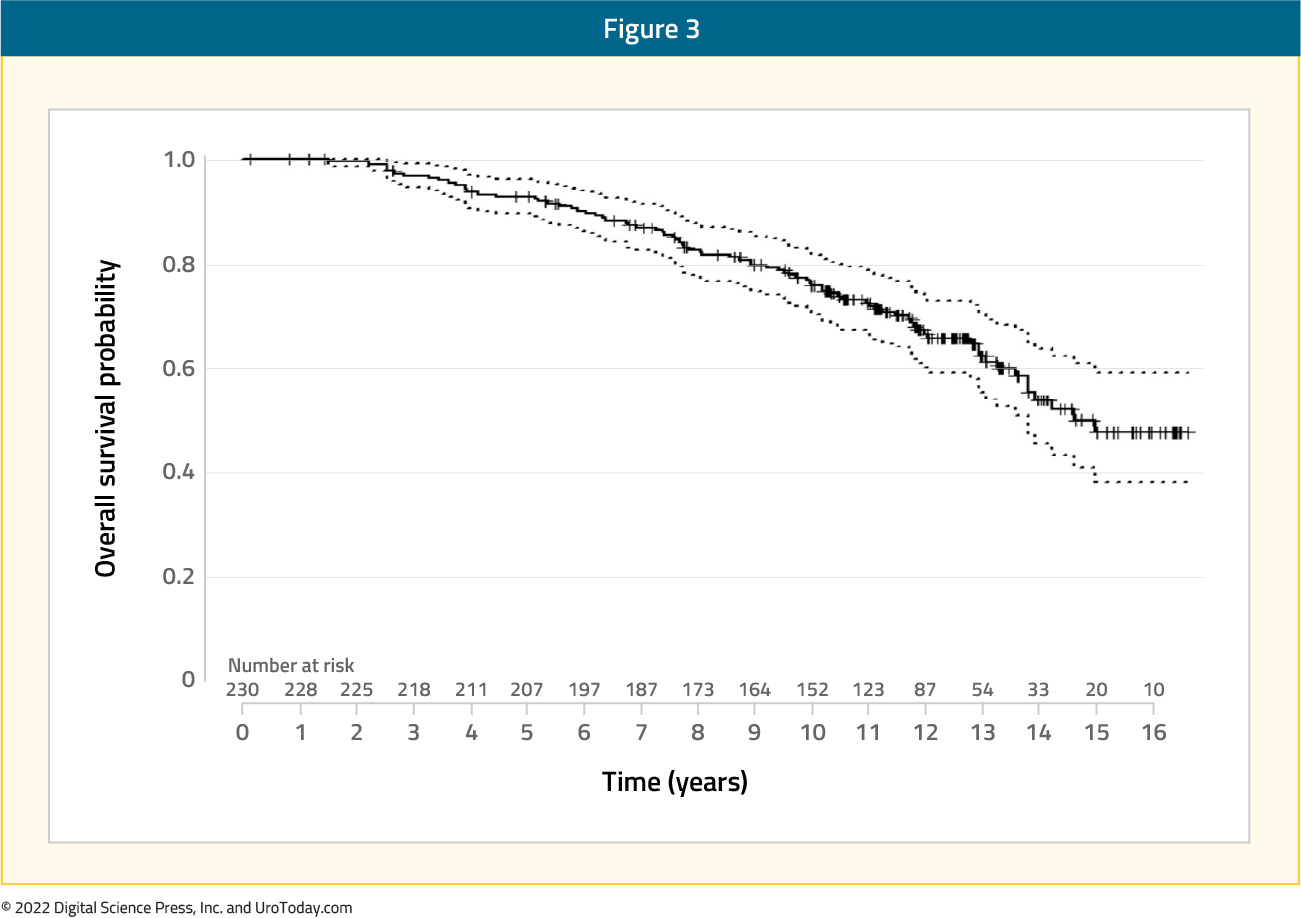
On multivariable analysis, the following variables were associated with worse overall survival:
- PSA nadir ≥ 0.01 ng/ml (HR: 5.2, 95% CI: 2.2 – 12, p < 0.001)
- ADT use ≤12 months (versus >24 months) (HR: 2.3, 95% CI: 1.3 - 3.9, p = 0.004)
The authors concluded that elective nodal pelvic irradiation with simultaneous hypofractionated prostate boost, along with long-term ADT, for high-risk prostate cancer patients is associated with promising 10-year biochemical control and an acceptable toxicity profile (5-year cumulative incidence of any late grade ≥3 gastrointestinal and genitourinary toxicity of 2.3% and 7.5%, respectively.)9
Given the increased utilization of ultrahypofractionation for treatment of prostate cancer in the primary prostate bed, what is the toxicity associated with elective nodal ultrahypofractionation? At ASTRO 2021, Dr. Glicksman presented pooled results from four prospective phase II trials of elective nodal irradiation using ultrahypofractionated radiotherapy that included patients with unfavorable-intermediate and high-risk prostate cancer. Radiotherapy was administered to the pelvic lymph nodes with nodal clinical target volume contouring according to the 2009 RTOG atlas and a planned target volume of 6 mm. The nodal clinical target volume dose was 25 Gy in 5 fractions whereas the planned target volume was 23.25 Gy in 5 fractions. Among the four included trials, different primary therapy regimens were employed, including hypofractionated external beam radiotherapy in 5 fractions with or without MR boost or HDR boost. In each study, the primary outcome was acute toxicity while secondary endpoints included late toxicity, patient-reported outcomes, and oncologic outcomes. Among 165 included patients, the median follow-up was 38 months (IQR: 10 - 63 months) and the majority of patients (59%) had high-risk disease. At baseline, most men had minimal urinary symptoms (62% with IPSS 0-9). The worst acute genitourinary and gastrointestinal toxicities respectively were 48% and 7.5% for grade 2, and 2.7% and 0% for grade 3. Cumulative incidence of late grade 2+ genitourinary and gastrointestinal toxicities at 36 months were 58% and 11.3% and for late grade 3+ toxicities were 1% and 0%, respectively. No grade 4+ acute or late toxicities were observed:
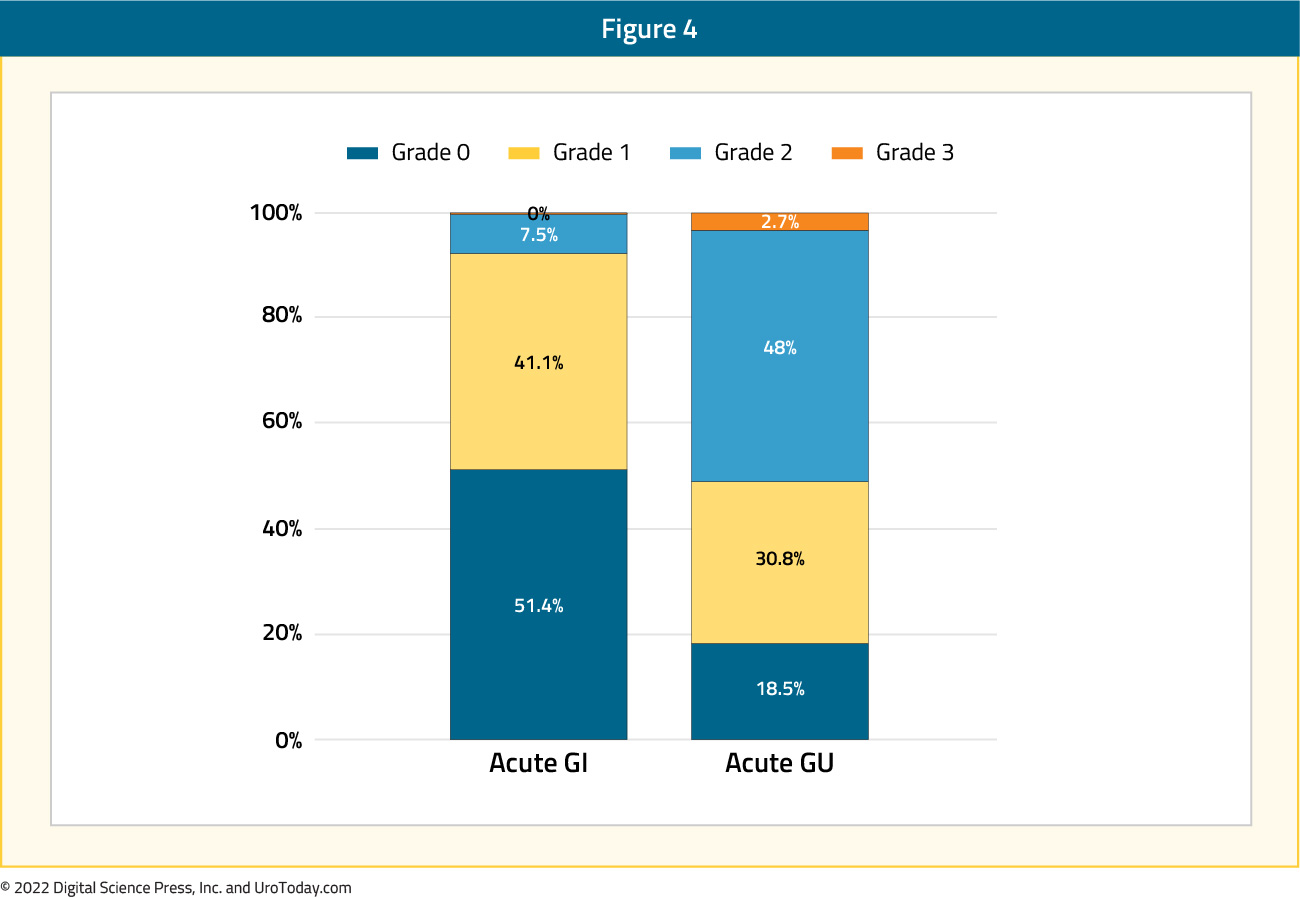
Using patient-reported EPIC scores, the authors found that bowel and sexual toxicity significantly worsened up to 1-year compared to baseline. The 3-year biochemical recurrence-free, metastasis-free, and overall survival rates were 98%, 98%, and 96%, respectively. The authors concluded that elective nodal irradiation using ultrahypofractionated radiotherapy is associated with a low incidence of grade 3+ toxicity, while grade 1-2 acute genitourinary and gastrointestinal toxicity is common.10
Similarly, a population-based prospective analysis of this question using the CEASAR study cohort recently demonstrated no clinically important differences in urinary incontinence, urinary irritative symptoms, bowel function, sexual function, or hormonal function between those patients who received prostate only or whole pelvis radiotherapy. There were no differences seen in more general measures of health-related quality of life either.11
Focal Boost to the Intraprostatic Tumor
Much as radical prostatectomy treats the entire prostate, to date, external beam radiotherapy has traditionally treated the entire prostate with relatively homogenous dosing. However, in keeping with a principle concordant with the idea of focal therapy, there is increasing interest in delivering an increased radiotherapy dose to the intraprostatic tumor.
Published in the Journal of Clinical Oncology in 2021, FLAME (Focal Lesion Ablative Microboost in Prostate Cancer) evaluated the role of additional focal boost to the MRI-visible lesion. In this randomized phase III trial, 571 patients with intermediate and high-risk prostate cancer received standard treatment at 77 Gy (2.2 Gy/fraction) to the entire prostate, with patients in the experimental arm receiving an additional simultaneous integrated focal boost up to 96 Gy (2.7 Gy/fraction) to the intraprostatic lesion visible on MRI. Organ at risk constraints were prioritized over the focal boost dose. The primary outcome was 5-year biochemical disease-free survival. After a median follow-up of 72 months, biochemical disease-free survival was superior in the experimental arm (HR: 0.45, 95% CI: 0.28 – 0.71, p<0.001), with 5-year rates of 92% and 85%, respectively.
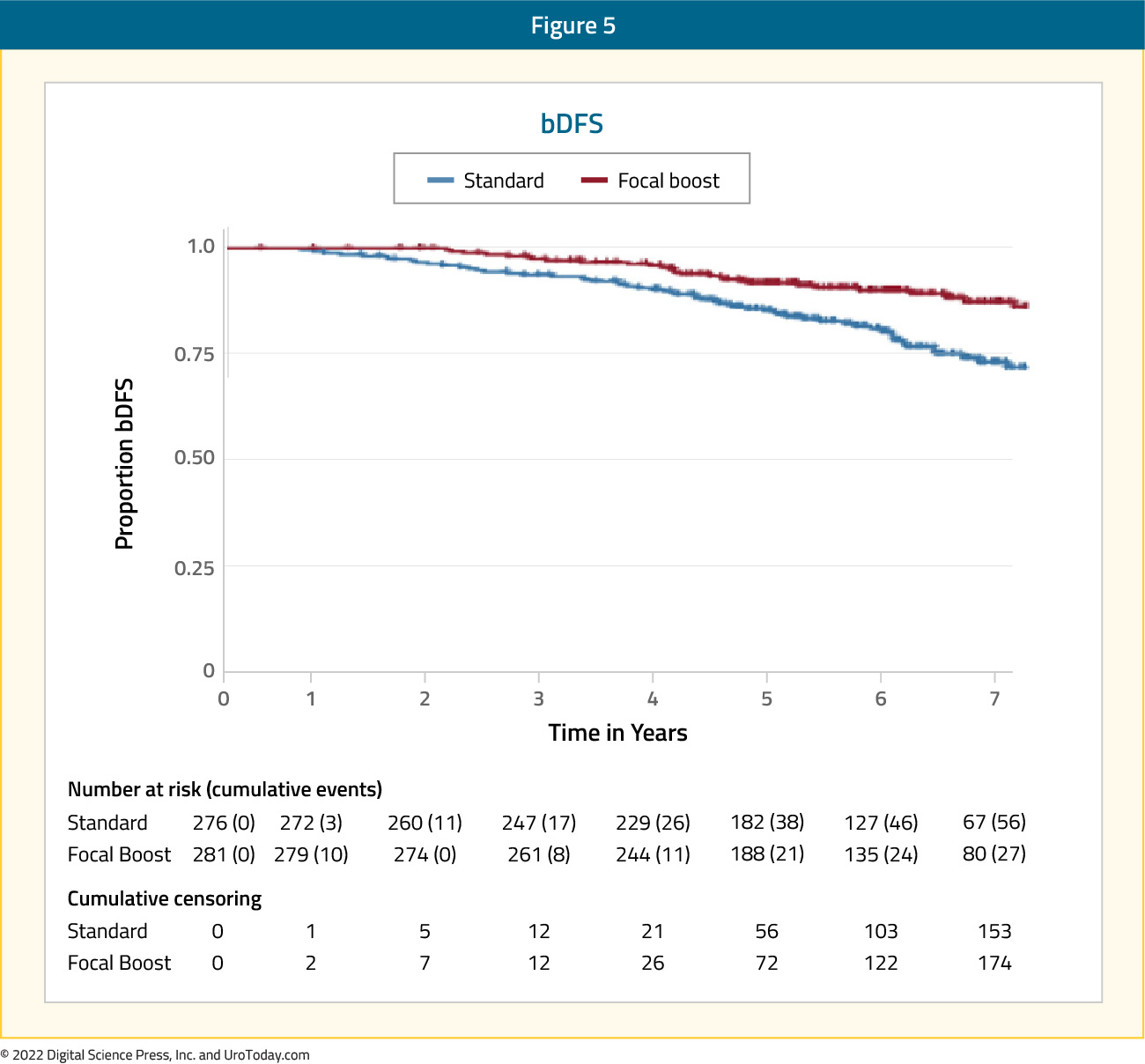
No difference was noted in the secondary, underpowered endpoints of prostate cancer-specific and overall survival. There were no significant differences in the cumulative incidence rates of grade 2+ late genitourinary (23% versus 28%) or gastrointestinal (12% versus 13%) toxicity between the standard and focal boost arms, respectively. Similarly, no difference in health-related quality of life was noted. The authors concluded that a high focal boost strategy to improve tumor control while respecting organ at risk dose constraints, is effective and safe.12
Conclusions
Together, these data suggest an increasing role for whole pelvis radiotherapy among patients harboring a high risk of occult micrometastatic disease. While trial data have suggested that oncologic benefits must be weighed against an increased risk of toxicity (particularly GI toxicity),13 observational data suggest that patient-reported outcome measures may not be dramatically affected. Localized intraprostatic dose escalation to MRI visible disease may provide an additional avenue of disease control.
Published Date: December 2022


