Introduction
Urothelial carcinoma of the upper tract accounts for only five to ten percent of all urothelial carcinomas,1 with an estimated annual incidence in Western countries of almost two cases per 100,000 inhabitants.2 Approximately two-thirds of patients with de novo upper tract urothelial carcinoma present with invasive disease at diagnosis,3 likely owing to the absence of a muscularis propria layer in the upper tracts. Large series have reported five-year cancer-specific mortality rates of 30% for muscle-invasive, organ-confined tumors and 56% for locally advanced tumors.4
Even when treated with extirpative surgery in the form of a radical nephroureterectomy, five-year survival rates for patients with invasive disease range from 39 to 60%.5 These outcomes strongly suggest the presence of micrometastatic disease at the time of diagnosis. Previously, the phase 3 POUT randomized clinical trial demonstrated a disease-free survival benefit for high risk patients receiving adjuvant chemotherapy after radical nephroureterectomy compared to those undergoing routine surveillance.6 Thus, it appears that these high risk patients are excellent candidates for early systemic therapy, perhaps ideally in the neoadjuvant setting given the expected decline in glomerular filtration rate post-nephroureterectomy, as well as post-operative morbidity that may delay time to systemic therapy receipt.
While neoadjuvant trials in patients with urothelial carcinoma of the bladder have demonstrated overall survival benefits of approximately 5% at five years,7 similar evidence in the upper tract disease space has been lacking largely owing to difficulty with patient recruitment secondary to the rarity of this disease process and the lack of care centralization. However, over the past decade, numerous phase II trials evaluating chemotherapy and immunotherapy agents in the neoadjuvant setting have emerged in this disease space. In this Center of Excellence article, we review the latest evidence for neoadjuvant systemic therapy options in patients with urothelial carcinoma of the upper tracts.
Accelerated Methotrexate, Vinblastine, Doxorubicin, and Cisplatin (MVAC)
Presented at ASCO 2014, Hoffman-Censits et al. presented the results of a prospective phase II multicenter study that evaluated three cycles of neoadjuvant accelerated methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) in patients with either cT204aN0-1 muscle invasive bladder cancer or upper tract urothelial cancer of the ureter or renal pelvis, with high-grade proven disease on biopsy or positive urine cytology with a mass on cross sectional imaging (NCT01031420).8 Patients received three cycles of accelerated MVAC (methotrexate 30 mg/m2, vinblastine 3 mg/m2, doxorubicin 30 mg/m2, cisplatin 70mg/m) day 1, pegfilgrastim 6 mg day 2 or 3, every two weeks. A radical nephroureterectomy, with lymph node dissection at the surgeon’s discretion, was performed 4-8 weeks after the last cycle. Ten patients were enrolled over a period of just under four years. Of the ten patients, six completed all three cycles of accelerated MVAC. The remaining four patients received <3 cycles due to grade 3 acute kidney injury (1), pyelonephritis and grade 3 diverticulitis (1), flare of underlying hepatitis (1), and grade 3 fatigue (1). Additional related grade 3 adverse events were anemia (1) and nausea/vomiting (1), with no grade 3-5 events reported. All patients underwent a radical nephroureterectomy within eight weeks of the last chemotherapy dose, at a median of 5.5 weeks. Pathologic responses were as follows:
- Complete response: 1/10
- <pT1 disease: 4/10
- pT2 disease: 2/10
- >pT3 or pN+: 3/10
In 2020, Margulis et al. published the results of a multicenter, prospective phase II trial (EA8141) that aimed to assess four cycles of neoadjuvant chemotherapy in patients with evidence of high-grade upper tract urothelial carcinoma. Patients with baseline creatinine clearance >50 ml/min would receive four cycles of accelerated MVAC, whereas those with a creatinine clearance of 30 to 50 ml or less would receive four cycles of gemcitabine/carboplatin. The study primary end point was a pathological complete response (ypT0N0). The study accrual goal was 30 patients per each arm and the trial schema was as follows: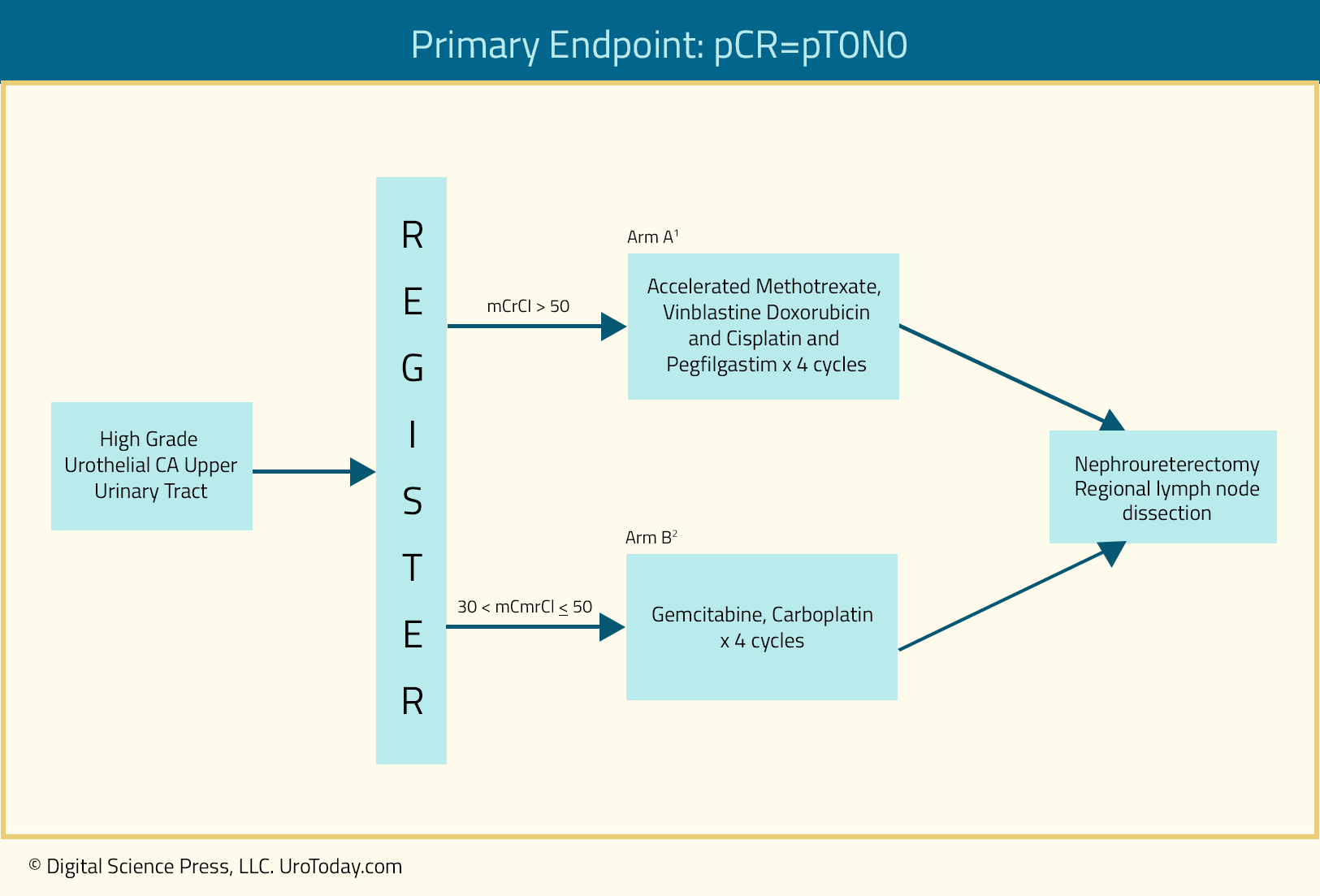
Between 2015 and 2017, 30 patients were enrolled into the accelerated MVAC arm, however only six patients were enrolled into the gem/carbo arm, which was subsequently closed due to poor accrual. Of the 29 eligible patients in the accelerated MVAC arm, 80% completed all planned treatments. A pathologic complete response was achieved in 13.8% of all patients, whereas 18/29 patients (62%) achieved a final pathologic stage of <ypT2. Conversely, in the gem-carbo arm, one patient (17%) achieved ypT0N0 at nephroureterectomy and 3/6 patients (50%) achieved a final pathologic stage of <ypT2.
The grade 3-4 toxicity adverse event rates were 23% and 50% in the accelerated MVAC and gem-carbo arms, respectively. There were no grade 5 toxicities encountered. In the accelerated MVAC arm, creatinine clearance decreased from a median of 82.0 mL/min at baseline to 75.5 mL/min post-chemotherapy and 48.0 mL/min post-surgery. The number of patients with a creatinine clearance of <60 mL/min increased from two at baseline, to six post-chemotherapy, and 20 post-surgery. Conversely, in the gem-carbo arm, creatinine clearance changed from a median of 45.5 mL/min at baseline to 48.1 mL/min post-chemotherapy and 38.7 mL/min post-surgery.9
Gemcitabine and Cisplatin
Presented at ASCO GU 2022, Yip and colleagues presented the final results of a multicenter, single arm, phase II trial of four cycles of neoadjuvant gemcitabine + cisplatin in patients with high-risk urothelial carcinoma of the upper tracts, based on either a high-grade biopsy result or imaging findings of cT2-4a disease and positive cytology. In January 2023, this study was subsequently published in the Journal of Clinical Oncology.10 All patients had no evidence of metastatic disease, an estimated glomerular filtration rate of at least 55 mL/min, and a Karnofksy performance status of at least 70%. The primary study endpoint was pathologic response rate (defined as < pT2N0).
Among 58 enrolled patients, 57 underwent surgery and were evaluable for the primary endpoint. Forty patients (70%) tolerated all four cycles of gem/cis and 89% of patients received at least three cycles. All patients proceeded to surgery. Thirty-six patients (63%, 95% CI 49 to 76%) demonstrated a pathologic response, meeting the primary endpoint of the study. A complete response was noted in 11 patients (19%), defined as ypT0N0. No patients experienced disease progression prior to surgery though seven (12%) had node positive disease at surgery. The 90-day ≥ grade 3 surgical complication rate was 7.0%.
Over a median follow-up of 3.1 years among survivors, two- and five-year progression free survival rates were 89% (95% CI: 81 to 98%) and 72% (95% CI: 59 to 87%), respectively:
Two- and five-year overall survival rates were 93% (95% CI: 86 to 100%) and 79% (95% CI: 67 to 94%), respectively. Pathologic complete and partial responses were associated with improved progression free- and overall survival compared with non-responders (>= ypT2N any; 2-year PFS 100% and 95% vs 76%, p < 0.001; 2-year OS 100% and 100% vs 80%, p < 0.001): 
Pembrolizumab
PURE-02 is a phase II trial that evaluated the use of three cycles of pembrolizumab 200 mg IV before radical nephroureterectomy in patients with high-risk upper tract urothelial cancer. This study included ten patients with clinical stage N0M0 upper tract urothelial carcinoma with high-risk features, defined by the presence of: high-grade disease, based on urinary cytology and/or biopsy, multifocal disease, ≥2cm tumor mass, and/or hydronephrosis. Patients with previous/concomitant bladder urothelial or variant histology were excluded. Between May 2018 and October 2020, ten patients were enrolled. Two had disease limited to the renal pelvis, six had a ureteral tumor, and a further two had both renal pelvis and ureteral tumors. Of the 10 patients, nine (90%) completed three cycles of pembrolizumab, with one patient (10%) dying due to the development of severe myasthenia gravis, myocarditis, myositis, and hepatitis after the first pembrolizumab course. The remaining patients did not show any grade 2-4 adverse events. In total, seven patients (70%) underwent a radical nephroureterectomy: one (14.3%) achieved a ypT1N0 response; the remaining patients were non-responders.
Results of this trial compare unfavorably to those seen with the PURE-01 trial of neoadjuvant pembrolizumab in patients with muscle-invasive urothelial carcinoma of the bladder, which demonstrated ypT0N0 and <ypT2N0 rates of 49.8% and 53.5%, respectively.11 As such, it does not appear that pembrolizumab holds promise as single agent therapy in the neoadjuvant setting prior to a radical nephroureterectomy for patients with high-grade upper tract urothelial carcinoma.
As follows is a table summarizing the pathological outcomes of phase 2 trials assessing neoadjuvant systemic therapy for patients with upper tract urothelial carcinoma:
Conclusions
Despite the relatively low incidence of upper tract urothelial carcinoma and the subsequent difficulties with recruiting patients for adequately powered clinical trials, it appears that neoadjuvant systemic therapy in patients with high-risk features achieves pathologic complete responses in the 10 to 20% range. While patients with pathologic responses have improved oncologic outcomes, predictive biomarkers that allow for appropriate patient selection for neoadjuvant therapy are currently lacking. Future trials that prospectively compare neoadjuvant therapy followed by surgery versus upfront surgery +/- adjuvant therapy will be needed to better define the role of neoadjuvant therapy in patients with high-risk upper tract urothelial carcinoma.
Published: March 20, 2023


