(UroToday.com) The 2022 ASCO annual meeting featured an oral abstract session on kidney and bladder cancer, including a presentation by Dr. Karim Chamie discussing the final results of the trial assessing IL-15RαFc superagonist N-803 with BCG in BCG-unresponsive CIS and papillary NMIBC. Patients with NMIBC CIS unresponsive to BCG have limited treatment options. N-803 (Anktiva) is a mutant IL-15-based immunostimulatory fusion protein complex (IL15RaFc) that promotes proliferation and activation of natural killer (NK) cells and CD8+ T cells, but not regulatory T cells. Phase 1b data in BCG-naïve patients with NMIBC demonstrate that intravesical administration of N-803 with BCG induced complete response in all patients, without recurrences for the study duration of 24 months. Pembrolizumab was approved in 2020 with a 41% complete response rate in a single arm phase 2 trial of 96 patients.1 At ASCO 2022, Dr. Chamie and colleagues report data on 161 subjects from an open-label, 3 cohort multicenter study (QUILT 3.032) of intravesical BCG plus N-803 in patients with BCG-unresponsive high-grade NMIBC (NCT03022825).
All treated patients received intravesical N-803 plus BCG, consistent with the standard induction/maintenance treatment schedule. The primary endpoint for Cohort A (CIS) is incidence of complete response of CIS at any time. The primary endpoint for Cohort B (Papillary) is disease-free rate at 12 months. The trial design for QUILT 3.032 is as follows:
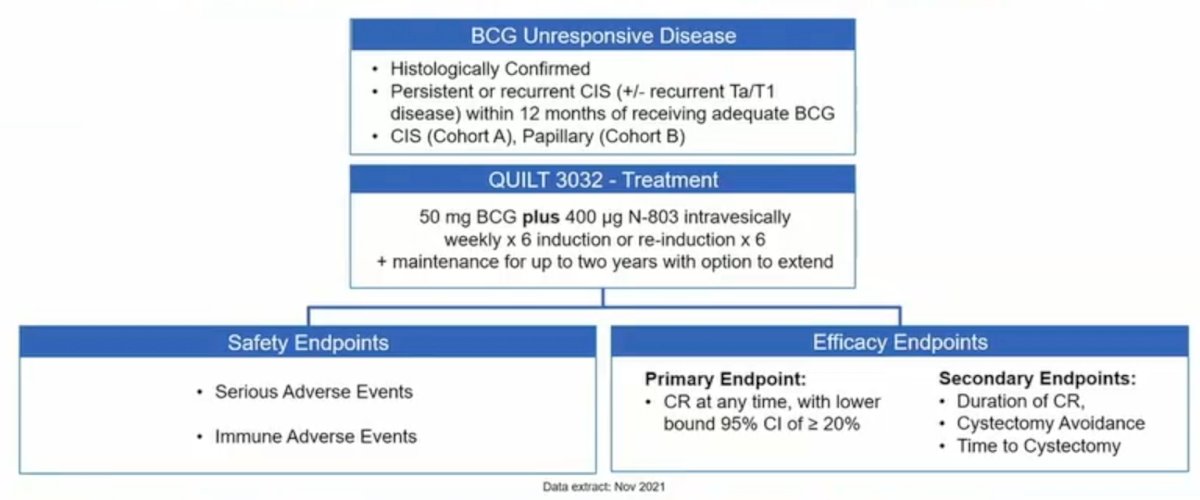
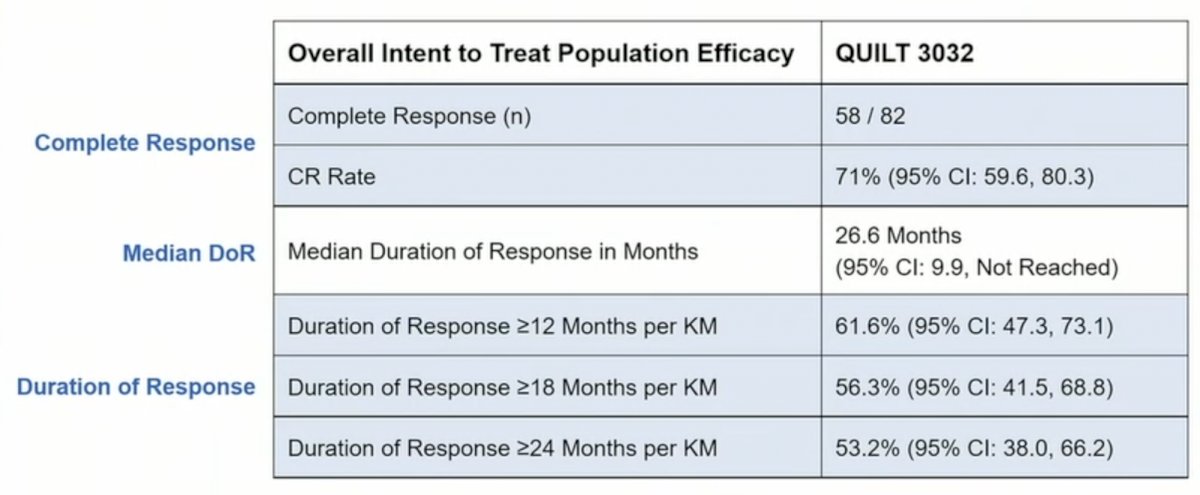
To date, there were 161 patients (84 CIS, 77 papillary) enrolled in this trial. In the overall population, median age was 72.3 years, 81% were male, the mean number of prior TURBTs was 4, and the median number of prior BCG doses was 12. Among CIS patients, the complete response rate was 71% (95% CI 59.6, 80.3), with a mediation duration of complete response of 26.6 months (95% CI 9.9 – not reached) in responders, 91% avoided cystectomy, and 96% had a 24-month bladder cancer specific progression free survival (defined as progression to MIBC):
Dr. Chamie then provided the following summary comparing results of N-803 to pembrolizumab in KEYNOTE-057:
- Complete response rate in CIS disease: N-803: 71% vs pembrolizumab: 41%
- Complete response rate in CIS disease, US population: N-803: 71% vs pembrolizumab: 29%
- Complete response rate in CIS/HT Ta at baseline: N-803: 81% vs pembrolizumab: 29%
- Complete response rate in CIS/T1 at baseline: N-803: 67% vs pembrolizumab: 42%
- Median duration of complete response: N-803: 26.6 months vs pembrolizumab: 16.2 months
- Cystectomy rate: N-803: 15.8% vs pembrolizumab: 41.6%
- Cystectomy rate after initial complete response: N-803: 9% vs pembrolizumab: 28%
- >= 30% complete response rate at 12 month assessment: N-803: 45% vs pembrolizumab: 20%
- >= 25% complete response rate at 18 month assessment: N-803: 33% vs pembrolizumab: 13%
Papillary patients have a 57% 12 month disease free survival rate, 48% 24-month disease-free survival rate, and 95% avoided cystectomy:
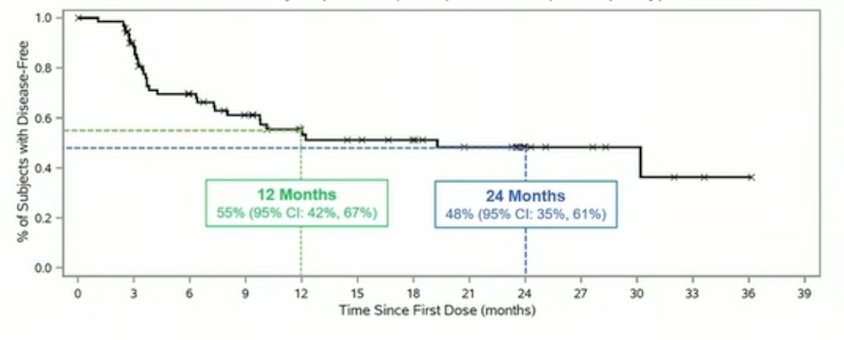
Median time to cystectomy in responders (n = 4) is 12.9 months versus 7.8 in non-responders (n = 8) for a 5.1-month delay in cystectomy. PK data shows no systemic levels of N-803, as activity is confined to the bladder. Low grade treatment related adverse events (grade 1-2) include dysuria (22%), pollakiurua (19%), hematuria (18%), fatigue (16%), and urgency (12%), all other adverse events were seen at 7% or less. No treatment related grade 4 or 5 adverse events were seen. No symptomatic adverse events were considered treatment related. No immune related symptomatic adverse events have been seen.
Dr. Chamie concluded his presentation discussing final results of the trial assessing IL-15RαFc superagonist N-803 with BCG in BCG-unresponsive CIS and papillary NMIBC with the following take-home messages:
- In these patients with BCG-unresponsive NMIBC, there is a 99% bladder cancer specific overall survival at 2 years
- In CIS patients 71% complete response rate with 26.6 months median duration of response, and 53% disease-free survival rate at 18 months in papillary disease
- Cystectomy was avoided >90% of patients with 2 years of follow-up
- The efficacy and safety profile of N-803 + BCG exceeds that of other available intravesical and systemic options for BCG-unresponsive NMIBC
Presented by: Karim Chamie, MD, Department of Urology, University of California-Los Angeles, Los Angeles, CA
Co-Authors: Sam S. Chang, Mark Gonzalgo, Eugene V. Kramolowsky, Wade J. Sexton, Paul Bhar, Sandeep K. Reddy, Patrick Soon-Shiong
Affiliations: Vanderbilt School of Medicine, Nashville, TN, University of Miami, Miami, FL, Virginia Urology Center PC, Richmond, VA, Department of Genitourinary Oncology, H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL, ImmunityBio, Inc., Morrisville, NC, ImmunityBio, Culver City, CA, NantKwest, Inc, Culver City, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 3 – Mon, June 7, 2022.
References:


