(UroToday.com) The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain was host to a presidential symposium of practice-changing trials. Professor Silke Gillessen presented the initial results of EORTC-GUCG 1333/PEACE-3, a phase III trial of radium-223 plus enzalutamide in asymptomatic or mildly symptomatic patients with bone metastatic castrate-resistant prostate cancer (mCRPC).
Abiraterone and enzalutamide are standard of care options for the 1st line treatment of patients with mCRPC progressing on androgen deprivation therapy.1,2 Radium-223 dichloride (223Ra) is an alpha particle-emitting calcium mimetic that selectively targets bone metastases and induces double-stranded DNA breaks.3 In the ALSYMPCA trial, 223Ra improved overall survival (HR: 0.7) in a treatment era prior to the introduction of abiraterone and enzalutamide.4
The ERA-223 trial previously tested the combination of abiraterone plus 223Ra versus abiraterone plus placebo. Not only did this combination not improve both symptomatic skeletal event-free survival and overall survival, but it was also associated with an increased rate of fractures.5
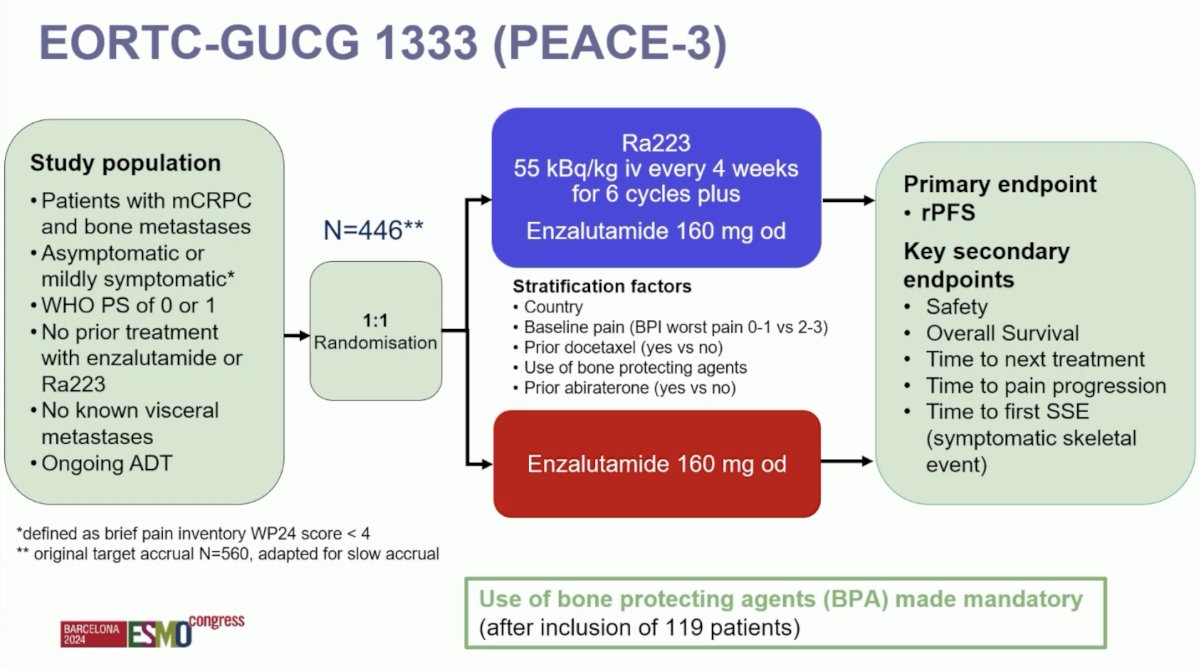
EORTC-GUCG 1333 (PEACE-3) is a prospective phase III trial of mCRPC patients with bone metastases who were asymptomatic or mildly symptomatic. Eligible patients had not received prior enzalutamide or 223Ra and had no known visceral metastases. Prior treatment with abiraterone and docetaxel in the hormone-sensitive setting was permissible. Study participants underwent 1:1 randomization to:
- Experimental arm: 223Ra 55 kBq/kg intravenously every 4 weeks for 6 cycles + enzalutamide 160 mg orally once daily
- Control arm: Enzalutamide 160 mg orally once daily
The primary endpoint was radiographic progression-free survival. Key secondary endpoints included:
- Safety
- Overall survival
- Time to next treatment
- Time to pain progression
- Time to first symptomatic skeletal event
Given the increased rate of skeletal fractures reported in the ERA-223 trial, a study amendment was introduced following the enrollment of the initial 119 patients to mandate the use of bone-protecting agents.
The study was powered to assess radiographic progression-free survival as the primary endpoint, with the statistical design and calculations summarized below:

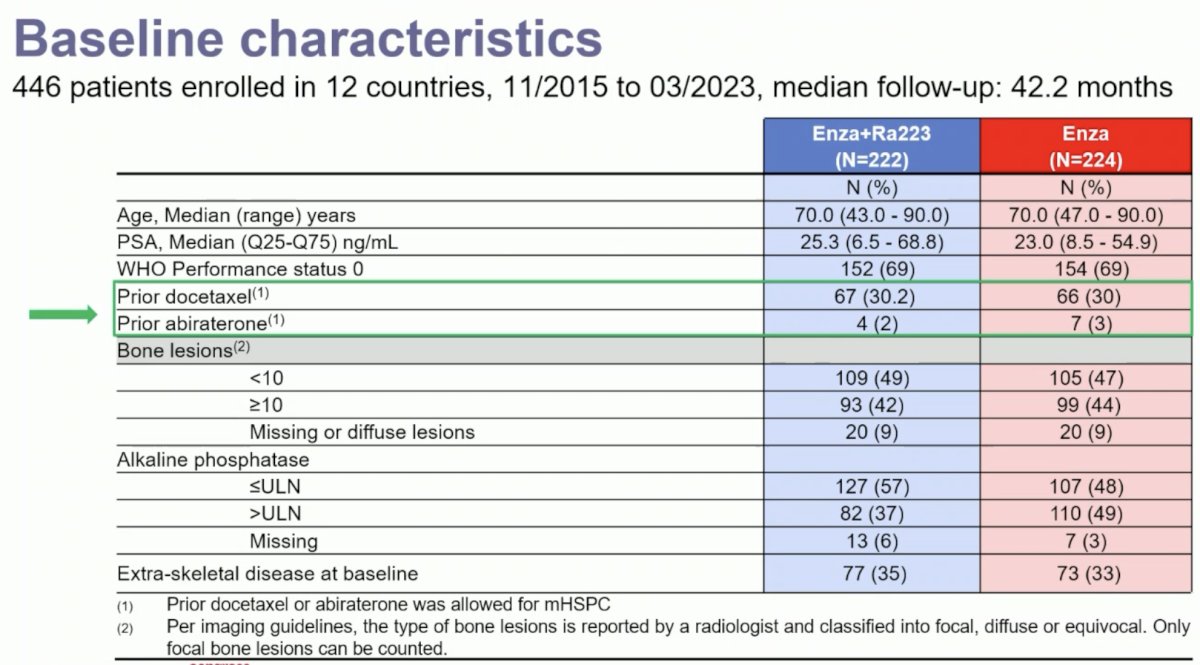
The baseline patient characteristics are summarized below. Between November 2015 and March 2023, 446 patients were enrolled from 12 countries. The median study follow-up was 42.2 months. The median patient age was 70, and the median PSA was 23–25 ng/ml. ~30% of patients had received prior docetaxel in the hormone-sensitive setting, and only 2–3% had received prior abiraterone.
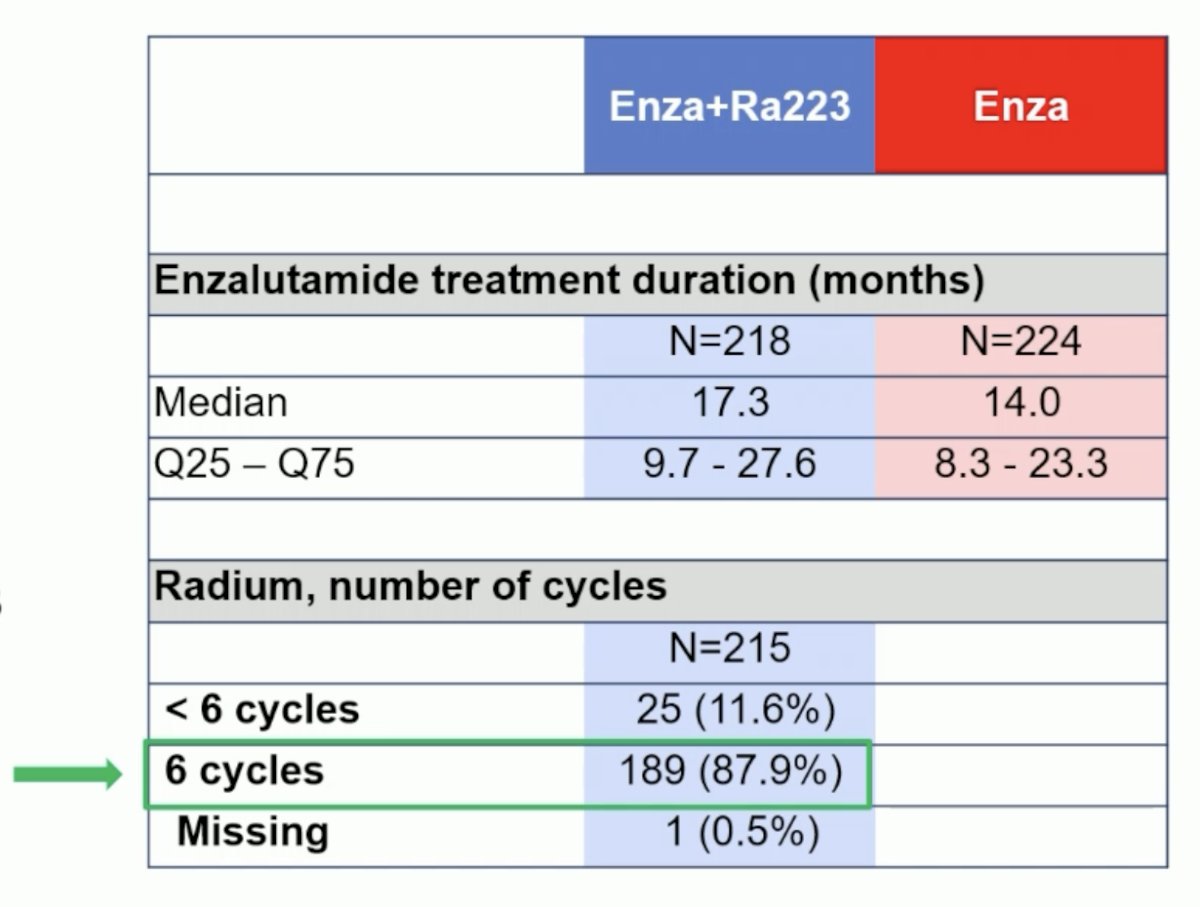
Of the 222 patients in the 223Ra + enzalutamide arm, 215 (97%) received both treatments. Of the patients who received 223Ra, 88% completed all 6 planned cycles.
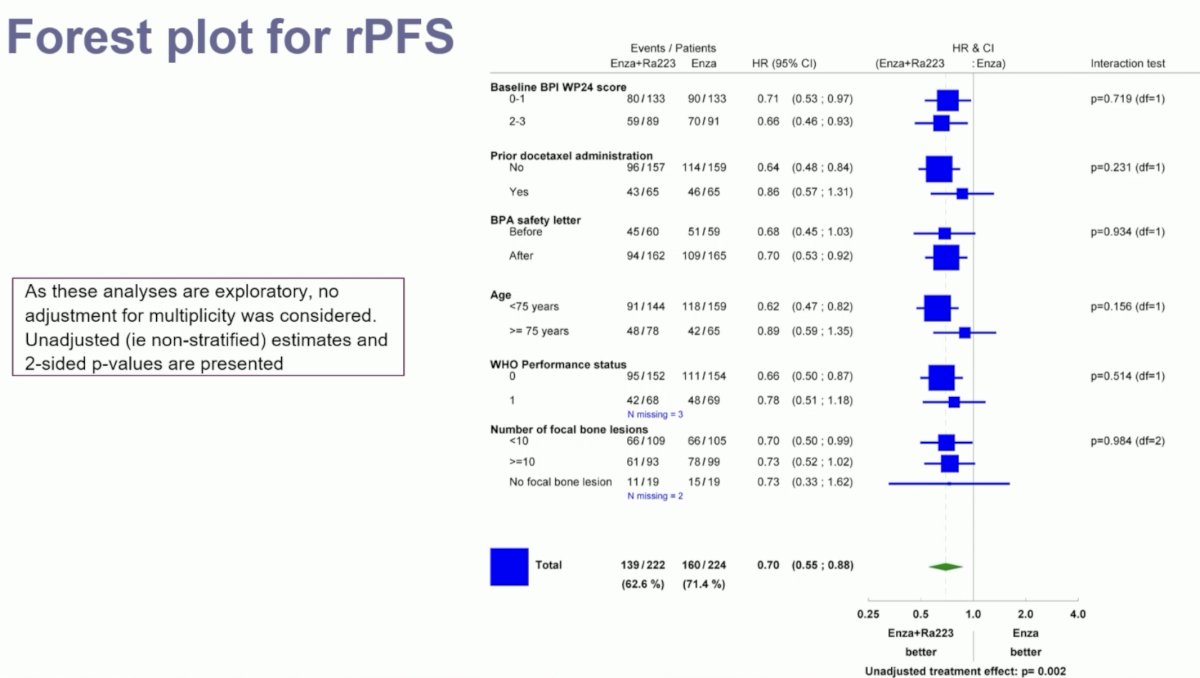
The addition of 223Ra to enzalutamide was associated with significant improvements in the primary endpoint of radiographic progression-free survival (median: 19.4 versus 16.4 months; HR: 0.69, 95% CI: 0.54–0.87, p=0.0009). At 24 months, 45% of patients in the 223Ra combination arm were free of radiographic progression, compared to 36% of patients in the enzalutamide monotherapy arm.

Subgroup analyses demonstrated consistent radiographic progression-free survival benefits in favor of combination 223Ra + enzalutamide.
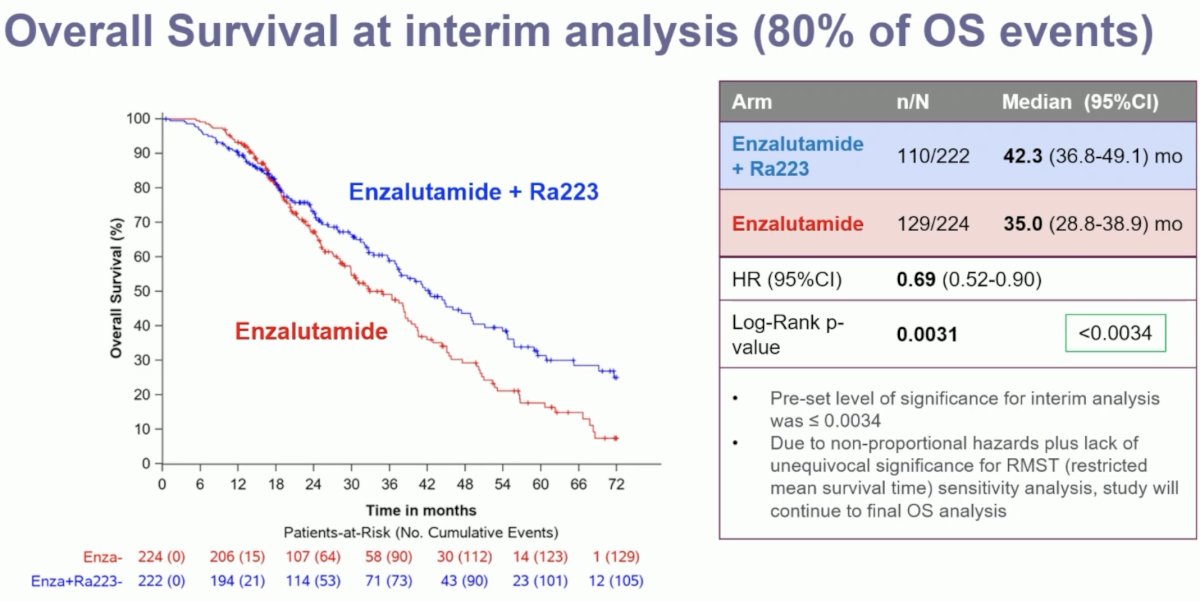
An overall survival benefit was observed with a combination 223Ra + enzalutamide. The median overall survival was 42.3 months versus 35 months for enzalutamide monotherapy (HR: 0.69, 95% CI: 0.52–0.90, p=0.0031). While the p-value was below the pre-specified α threshold of 0.0034, there was evidence of non-proportional hazards (i.e., the curves cross during initial follow-up). Given this plus the lack of unequivocal significance for restricted mean survival time sensitivity analysis, the study will continue to the final overall survival analysis.
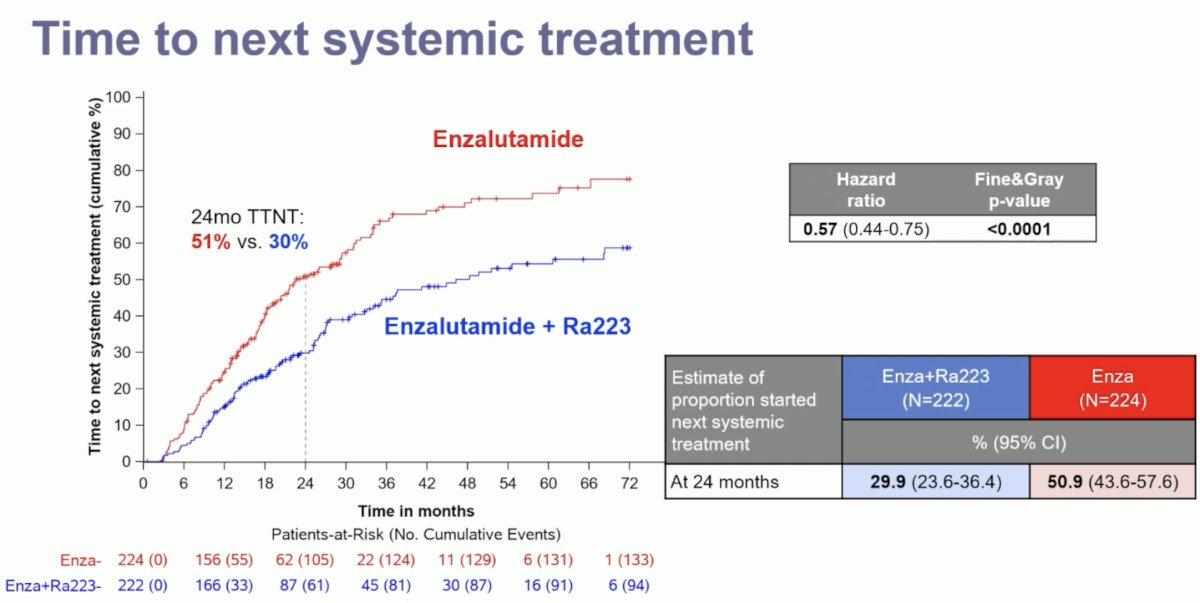
Time to next systemic treatment was also improved with the 223Ra + enzalutamide combination, with only 30% of patients in this treatment arm requiring a next-line systemic treatment at 24 months follow-up, compared to 51% of patients in the enzalutamide monotherapy arm (HR: 0.57, 95% CI: 0.44–0.75, p<0.0001).
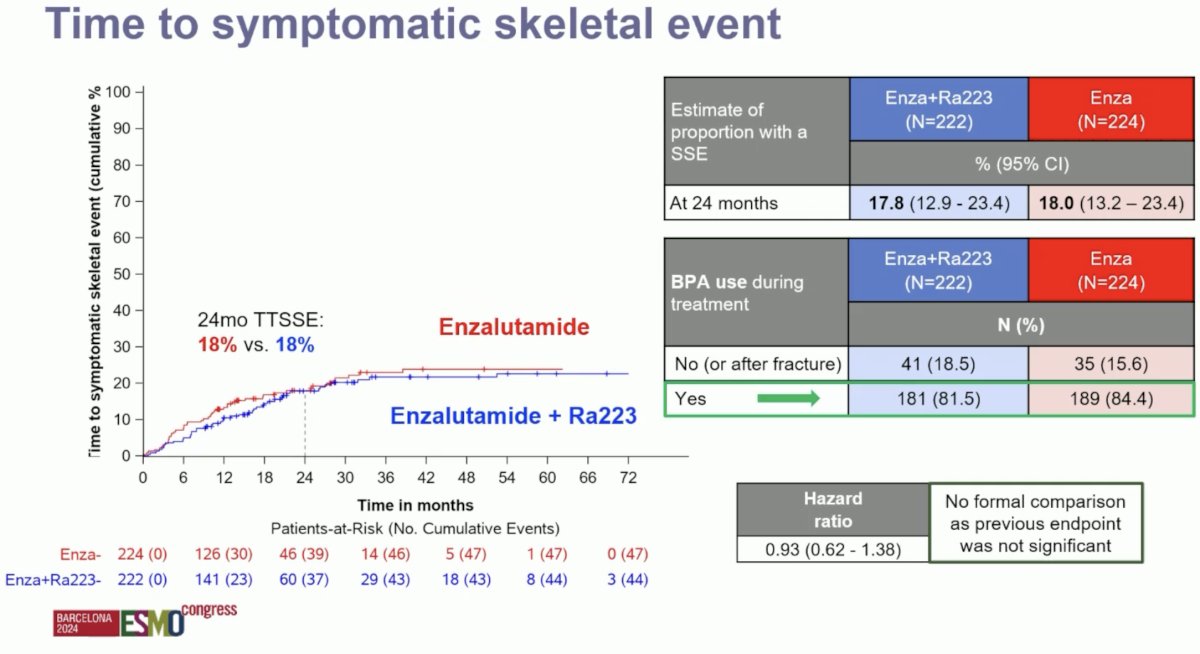
Reassuringly, there were no differences in time to symptomatic skeletal event, with 18% of patients in both arms having an event by 24 months follow-up. Prior to implementing the study amendment, only ~50% of patients were using bone protecting agents. At the end of enrolment, 82–84% of patients were using bone protecting agents.
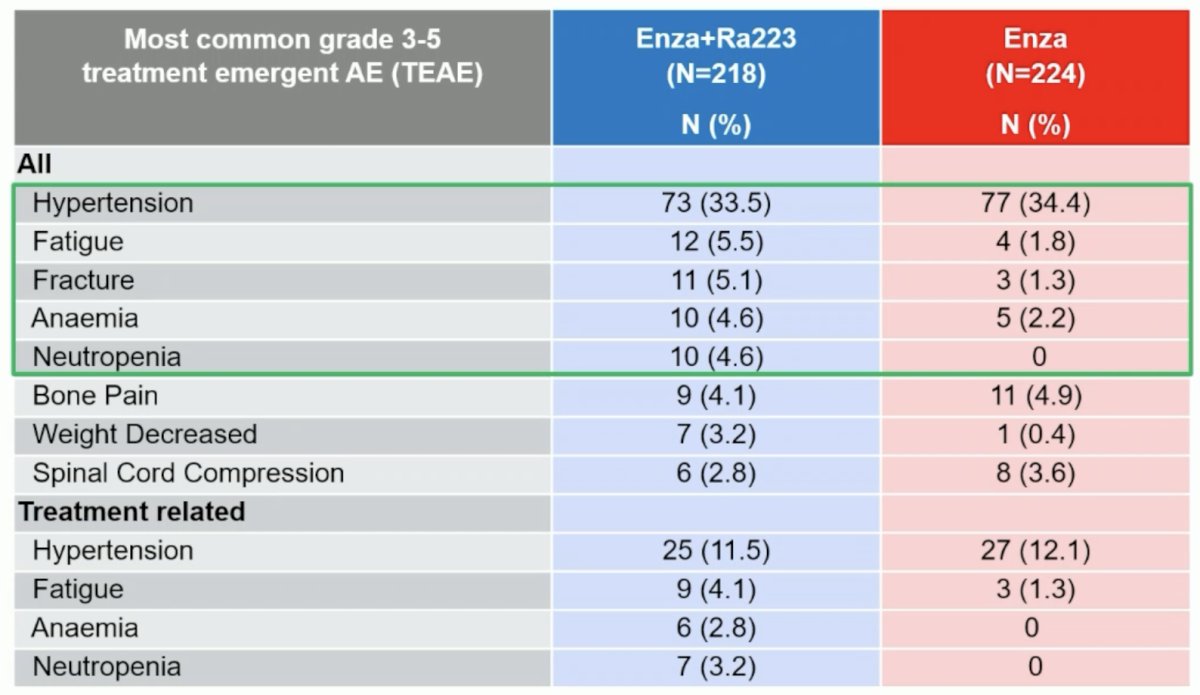
From a safety standpoint, grade ≥3 drug-related adverse events were observed in 28% and 19% of patients in the experimental and control arms, respectively. Deaths due to adverse events were observed in 3% and 2% of patients, respectively, none specifically related to the drugs used.

The most common grade ≥3 adverse events in the 223Ra + enzalutamide arm were hypertension (34%), fatigue (5.5%), and fractures (5.1%).
Dr. Gillessen concluded her presentation of the PEACE-3 trial as follows:
- The combination of enzalutamide and 6 cycles of 223Ra shows a statistically significant improvement in radiographic progression-free survival
- Hazard ratio of 0.69 (p=0.0009)
- The median radiographic progression-free survival increased from 16.4 months with enzalutamide to 19.4 months with this combination
- The improvement in radiographic progression-free survival is supported by a significantly improved overall survival (HR 0.69, p=0.0031)
- Due to non-proportional hazards, this will be tested further in the final overall survival analysis to confirm and further characterize the result
- Improvement in radiographic progression-free survival is also supported by a statistically significant improvement in time to next systemic treatment (HR: 0.57, p<0.0001)
- Drug related grade ≥3 adverse events increased from 19% to 28% in the combination arm
- These results support the combination of enzalutamide plus 223Ra (plus a bone protecting agent) as a potential new first line mCRPC treatment option for patients with prostate cancer and bone metastases who have not received a prior androgen-receptor pathway inhibitor
Presented by: Silke Gillessen Sommer, MD, Head of the Medical Oncology Department and Medical Scientific Director of the Oncology Institute of Southern Switzerland (IOSI) at the Ospedale San Giovanni in Bellinzona, Switzerland
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
Related content: PEACE-3 Trial Results: Radium-223 + Enzalutamide in mCRPC - Bertrand Tombal
References:
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013; 368(2):138-48.
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424-33.
- Morris MJ, Corey E, Guise TA, et al. Radium-223 mechanism of action: implications for use in treatment combinations. Nat Rev Urol. 2019; 16(12):745-56.
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369(3):213-223.
- Smith M, Parker C, Saad F, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): A randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019; 20(3):408-19.


