Introduction
The coronavirus disease (COVID-19) caused by novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a highly contagious disease with possible origin in the wet animal market at Wuhan in the Hubei province of China.1 The World Health Organisation declared COVID-19 as a pandemic on March 11, 2020.2 Patients who are on cancer treatment, immunosuppressive medications, bone marrow transplantation and those with human immunodeficiency virus infection appear to have increased morbidity and mortality from COVID-19.3-5
Prioritizing health care is extremely important in this desperate situation. The effect of COVID-19 on the outcome of urological cancers and vice versa is unknown. As the medical fraternity deals with frontline management of COVID-19, urologists can reduce unnecessary utilization of the medical facilities already strained by COVID-19. This calls for effective triage of urological services in the country. Although redistribution of urological services is the need of the hour, there is also an enormous concern on the effect it has on diagnosis and treatment of cancers.6 This review aims to summarize the evidence available for safe management of urological cancers in the COVID-19 pandemic. However, considering the limited literature available, readers are advised to be cautious on generalized statements in this review. Regional and institutional protocols should be strongly adhered to in patient management during the pandemic.
Materials and Methods
A literature search was made in MEDLINE with search items, including coronavirus, COVID-19, urological cancers, kidney or renal cancers, prostate cancer, bladder cancer, testicular cancer, and penile cancer. Studies between December 2019 and April 2020 were included for the review. The recommendations for the optimal management of urological cancers as suggested by the American Urological Association, European Association of Urology, British Association of Urological Surgeons, and the Cancerology Committee of the French Association of Urology were also referred.
SARS-CoV-2 Virus and COVID-19
The SARS-CoV-2 is a novel coronavirus consisting of positive single-stranded ribonucleic acid (RNA) and four proteins – S (spike), E (envelope), M (membrane), and N (nucleocapsid).7 The virus attaches itself to the angiotensin converting enzyme-2 (ACE2) on the surface of pneumocytes. This enzyme is an antioxidant which protects against lung injury. This protective effect is impaired in diabetic patients. On the other hand, this enzyme is overexpressed in patients on ACE inhibitors and angiotensin receptor blockers, making these two groups of patients more vulnerable to the effects of the virus.8 Bats are thought to be reservoirs of the novel coronavirus, and recently, pangolins have been identified as the intermediate hosts.9 Treatment of the disease is predominantly supportive; however, remdesevir, favipiravir, arbidol, chloroquine, and azithromycin have shown benefit in nonrandomized prospective and retrospective studies. Further trials are underway to test their safety and efficacy in COVID-19.10-13
Cancer Care in COVID-19 Pandemic
Cancer patients are considered as high risk in the COVID-19 crisis due to the mutually deleterious effect of cancer and COVID-19 on homeostasis. In a retrospective study, cancer patients diagnosed with COVID-19 had higher incidence of complications and mortality when they had received cancer therapy within 14 days before COVID-19 diagnosis. Among the 28 cancer patients with COVID-19 included in the study, 28.6% acquired the virus during the hospital stay.14
Liang et al. recommend postponing elective surgeries and adjuvant chemotherapy for stable cancers, providing personal protective equipment for cancer patients and escalating the surveillance and treatment of cancer patients with COVID-19.15 Moreover, the decision to treat malignancy in this pandemic must be made on a case to case basis.16 Equally important in this resource limited setting is the palliative care of terminally ill cancer patients.17
COVID 19 and Urological Malignancies
There is little evidence regarding the optimal management of urological cancers amidst the peak of COVID-19 pandemic as the data is in an elementary stage and the population is diverse. Hence, the general and specific measures mentioned below should not be considered final and local guidelines and hospital resources should be taken into consideration while managing urological cancers during the pandemic. Moreover, the diagnosis and treatment of COVID-19 in a patient with urological malignancy interferes with the oncological outcome and causes delay in the treatment.
General measures
The risk of delay in cancer diagnosis and treatment must be weighed against the risk of getting exposed to the virus in the hospital. COVID-19 screening must be done for all patients planned for surgical procedures as even asymptomatic individuals can harbor the novel coronavirus.18 Informed consent has to be obtained before planning any surgery, meticulously explaining the risk of nosocomial COVID-19 infection and its complications. It is also imperative to disclose the increased risk of perioperative adverse events aggravated by COVID-19 as well as the paucity of resources in the adequate management of such events.19 Patients should be counseled about the risks of adjuvant immunosuppressive cancer therapies which are generally deferred during the pandemic.18,20,21
In a retrospective study of 113 deceased COVID-19 patients, almost one-fourth (25%) developed acute kidney injury (AKI) due to overwhelming sepsis. Hence, in order to reduce the possibility of AKI in COVID-19 patients with obstructing urological cancers, relieving the obstruction should be given priority.22 COVID-19 patients are reported to have a higher incidence of deep vein thrombosis and ischemic episodes. Therefore, coagulation parameters should be checked preoperatively (D-dimer, prothrombin time, and platelet count in the order of importance).23 Bleeding is rare in COVID-19; however, the risk can be predicted from prothrombin time, platelet count and serum fibrinogen levels. The International Society of Thrombosis and Hemostasis recommends starting prophylactic low molecular weight heparin for all COVID-19 patients, unless there is significant bleeding or thrombocytopenia (platelets <25000/mm3 ).24 The treating physician should reduce the number of hospital visits for cancer patients on follow-up, and consult via telecommunication systems.25 The telemedicine society of India has laid down guidelines on telemedicine in India and is involved in training healthcare providers in this revolution of doctor–patient communication.27
Specific measures
Renal cancer
Diagnosis
All patients above 45 years of age presenting with persistent hematuria without evidence of urinary tract infection or signs of metastatic renal cell carcinoma should be completely assessed.18 Renal mass biopsy (RMB) is avoided given the lower availability of interventional radiology services.18 Considering the slow growth and low potential for metastasis, biopsy and further staging for suspected T1a and T1b renal tumors can be safely deferred with repeat imaging at 6–9 months and 3–6 months, respectively.18,27 However, further workup and staging can be performed for tumors larger than 7 cm (T2 onwards).18
Treatment
Localized renal cell carcinoma
Given the low growth rate which depends on initial tumor size, partial nephrectomy for T1a and T1b tumors can be safely deferred as long as 6–9 months and 3–6 months during the pandemic, without affecting the overall survival.27 However, evidence is lacking on the safety of delayed surgery beyond 6 months on overall survival. Ablative treatments should also be generally avoided during the pandemic.18 This is a reasonable recommendation considering the extremely slow growth kinetics and weak metastatic potential of these tumors.27,28 T2 tumors must undergo surgical management as per the department protocol; however, the surgery can be delayed as per the local availability of resources [Figure 1].18
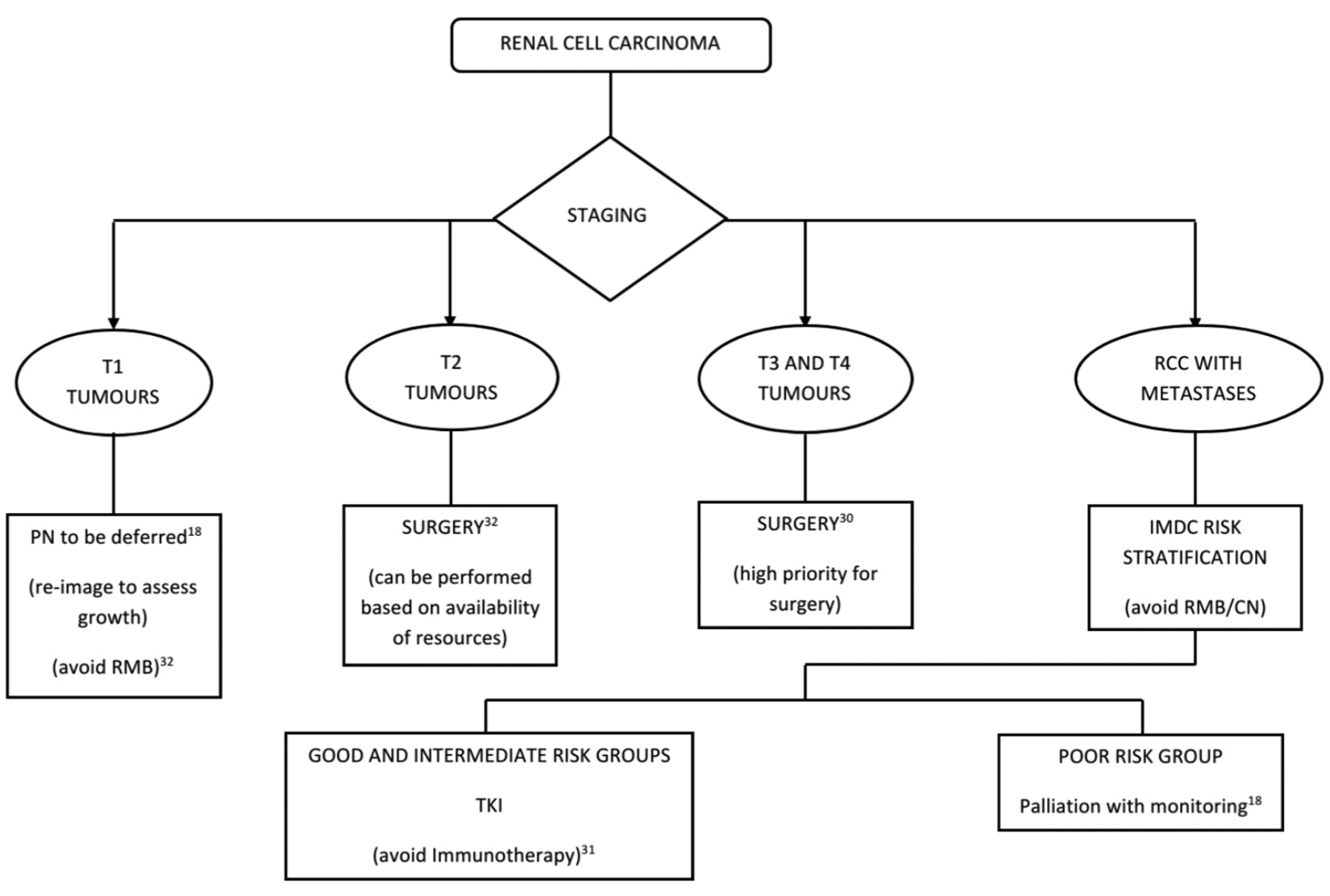
Figure 1: Algorithm for management of renal cell carcinoma in the COVID-19 pandemic. AS = Active surveillance, PN = Partial nephrectomy, RCC = Renal cell carcinoma, RMB = Renal mass biopsy, CN = Cytoreductive nephrectomy, TKI = Tyrosine kinase inhibitor
Locally advanced renal cell carcinoma
T3 tumors which are nonmetastatic get the highest priority for the surgical management in this pandemic setting and such patients need to undergo surgical management at the earliest.29,30
Metastatic renal cell carcinoma
It is prudent to avoid cytoreductive nephrectomy and RMB during the pandemic.18 Good risk individuals according to the International Metastatic RCC Database Consortium can be started on tyrosine kinase inhibitors (TKIs) either Sunitinib or Pazopanib and followed up through telemedicine.18 The same treatment (TKI) can be offered to asymptomatic intermediate-risk groups as immunotherapy cannot be initiated considering the immunomodulatory adverse effects and less availability of these drugs during the pandemic.18 Immunotherapy with immune checkpoint inhibitors such as anti-programmed cell death-1 (PD-1)/programmed cell death ligand-1 (PD-L1), and anti-cytotoxic T lymphocyte associated protein-4 (CTLA-4) has been linked to interstitial pneumonitis and cytokine release syndrome (CRS).31 The pathogenesis of COVID-19 is similar and overlaps these adverse events, and hence, a clinical synergism cannot be ruled out. Therefore, it is prudent to avoid immune checkpoint inhibitors in advanced renal cancer patients during the pandemic.31 Patients in the poor risk category can be considered for palliative treatment with surveillance alone, during the pandemic.18,32
Follow-up
Given the reduced availability of radiological services during the pandemic, the post-treatment radiological assessment of low/intermediate risk renal cancers can be delayed for 6 months and that of high risk renal cancers for 3 months.18
Bladder cancer and upper tract urothelial malignancy
Diagnosis
A complete workup for hematuria should be performed in patients below 45 years of age with visible hematuria in the reduced resources setting while an ultrasound will suffice for patients with microhematuria above 60 years of age.18 However, in severely restricted settings, the workup should be deferred in patients with microhematuria above 60 years and ultrasound alone to be considered initially for visible hematuria in patients below 45 years of age.18
Treatment
Shedding of the SARS-CoV-2 in the urine has not been documented in the majority of hospitalized COVID-19 patients till date.33 However, the persistence of viral RNA in urine has been documented in COVID-19 treated with steroids.34 Hematuria and minor mucosal bleeds associated with endourological manipulations increase the urinary viral load, and hence, endoscopic procedures should be kept to a minimum.34
Nonmuscle invasive bladder cancers
Transurethral resection of bladder tumor (TURBT) is cornerstone for the diagnosis of bladder cancer; however, it increases the risk of contamination of the theater and surgical team. Therefore, it can be deferred for 3 months in tumors which are deemed low risk-single, small, presumably low grade with no carcinoma in situ as evident by urine cytology.18,32 Perform TURBT for actively bleeding, solid, multifocal, and large tumors.18 Tumors with associated upper tract obstruction need to be prioritized for drainage procedures considering the risk of COVID-19 infection and its associated AKI.22 Post-TURBT intravesical instillations must be deferred if the agent is unavailable during the pandemic. Ambulatory postoperative intravesical instillations should be encouraged when possible, to clear beds required for COVID-19 patients.32 Recent retrospective studies point to a potentially protective effect of bacillus Calmette Guerin (BCG) vaccination against COVID-19 due to nonspecific immune benefits.35 Although intravesical BCG also induces a systemic immune response, its safety in patients with COVID-19 has not been confirmed.36 While maintenance BCG therapy is important, it is the induction course which confers maximum protection against progression and recurrence. Hence, it is oncologically safe to initiate BCG induction in the intermediate and high risk nonmuscle invasive bladder cancers (NMIBC) groups. Maintenance BCG instillations for intermediate and high risk groups can be safely delayed for 3 months followed by reassessment with a cystoscopy at the end of 3 months.37 In addition to reducing the number of hospital visits, this approach also avoids the rare instance of pulmonary manifestation of BCG toxicity which can mimic COVID-19.38 Perform a re-TURBT only when there is a strong suspicion of understaging. In case of severe restriction of resources, TURBT should be reserved only for actively bleeding bladder tumors.18,32
Follow-up cystoscopy should be deferred for 3 months in high-risk tumors, 6 months for intermediate risk and 12 months for low risk NMIBC.32 In severely restricted settings, surveillance for low risk tumors can be stopped and cystoscopy should be performed at 12 months and 6 months for intermediate and high risk groups, respectively.32 Cystoscopy should be preferably done in day care setting under local anesthesia.32
Muscle invasive bladder cancer
For better outcomes, radical cystectomy is recommended to be performed within 90 days of diagnosis of MIBC, based on local resource availability during the pandemic.18,30,32 Neoadjuvant chemotherapy (NACT) with growth factors should be offered to newly diagnosed MIBC. However, the immunosuppressive effect of NACT needs to be discussed with the patient and decision to be taken on a case to case basis.32 Hemostatic radiotherapy is an option for actively bleeding MIBC when radical cystectomy is deferred due to limited resources.18
Metastatic bladder cancer
Growth factors can be used along with chemotherapy to minimize the risk of immunosuppression.32 Immunotherapy regimens employing anti-PD-1/PD-L1 and anti-CTLA-4 agents should be avoided in bladder cancer considering the high risk of interstitial pneumonitis and CRS which are common with these agents and also in the late phases of COVID-19 [Figure 2].31
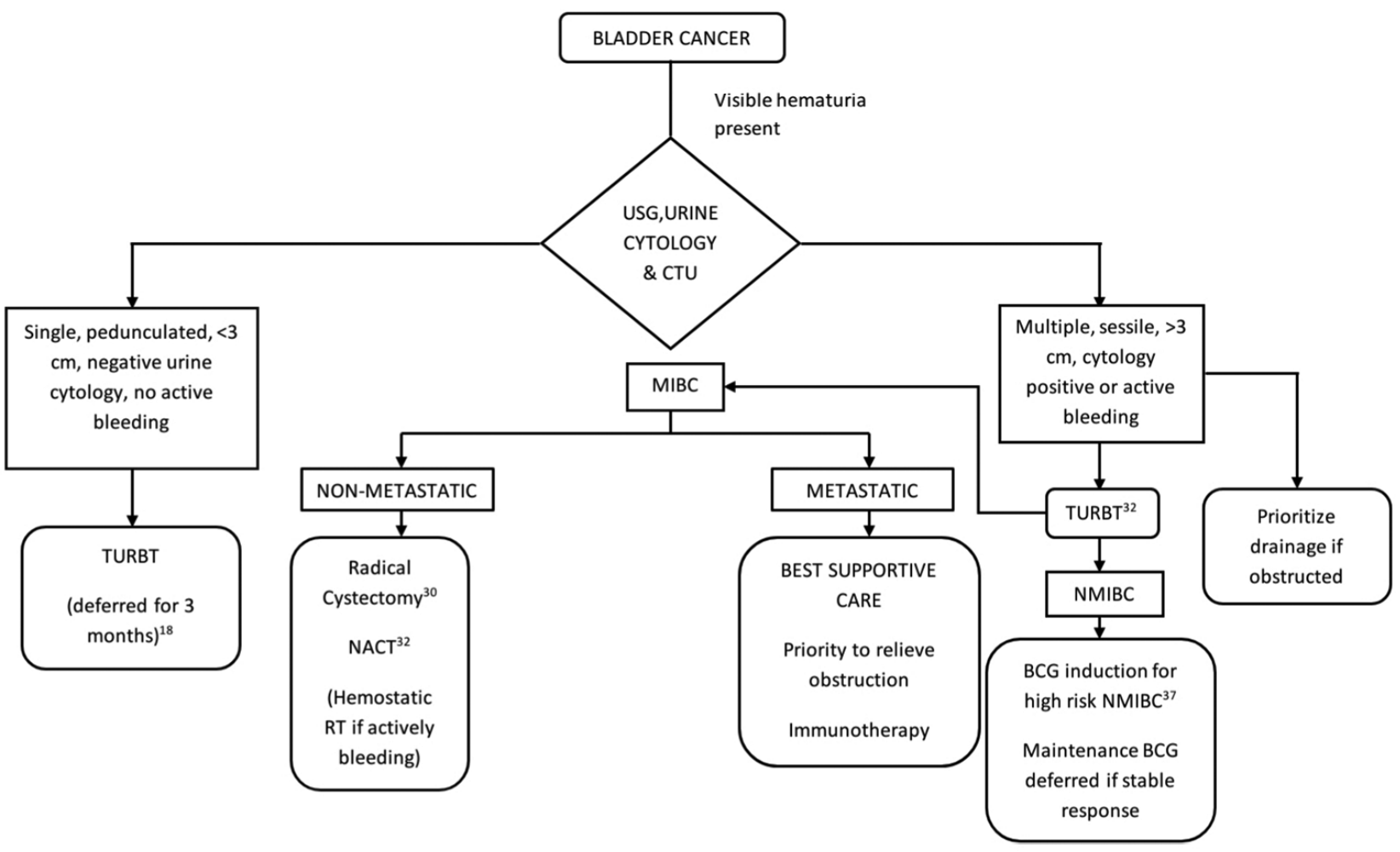
Figure 2: Algorithm for management of bladder cancer in the COVID-19 pandemic. USG = Ultrasonogram, CTU = Computed tomography with urogram, NACT = Neoadjuvant chemotherapy, G-CSF = Granulocyte colony stimulating factor, RT = Radiotherapy
Upper tract urothelial cancers
Radical nephroureterectomy should be advised for stable patients suspected of high grade disease or those who present with active hematuria.30 In severely restricted access to resources, surgery can be deferred and embolization can be considered for intractable hematuria.18 However, a surgical delay of more than 3 months has been associated with disease progression in invasive urothelial cancers.39
Prostate cancer
Diagnosis
Elderly and frail men with an elevated prostate specific antigen (PSA) may be advised to attend the clinic at a later date after the pandemic.18 Delay in diagnosis by 3 and 3–6 months does not affect the long term outcome in high and intermediate/low risk groups respectively.37 Therefore, prostate biopsy can be safely delayed for 3 months even in patients with risk factors for high risk prostate cancer (PSA >20 ng/ml, PSA doubling time <6 months, DRE suggestive of T3 disease, local or systemic symptoms).37 In men with none of these risk factors, it is prudent to delay the prostatic biopsy for 3–6 months without compromising oncological outcomes.37 Transperineal prostate biopsy is preferable over transrectal ultrasound guided biopsy considering the lower risk of post-biopsy complications and sepsis.37,40 Patients unwilling to visit the hospital due to the risk of contracting COVID-19 can be advised to repeat PSA after 3–6 months.32
Treatment
Low- and intermediate-risk localized prostate cancer
The length of waiting period for surgery is not crucial in low risk category. The maximal safe waiting time for surgery is 60 days in case of the intermediate risk group.39 Hence, radical prostatectomy can be safely delayed in these risk groups in the pandemic setting.30 Active surveillance (AS) can also be delayed and magnetic resonance imaging and prostate biopsy as part of AS protocols can be postponed during the pandemic, given the limited availability of radiological services.32
High-risk localized prostate cancer
A delay of surgery for more than 28 days has negative consequences in outcome of high risk prostate cancer.39 Hence, the high risk group should be prioritized for radical prostatectomy as part of multi-modality therapy (MMT) whenever local resource availability is conducive.18,30,32 However, COVID-19 patients who undergo surgery have a mortality rate of 20.5%, and hence, radical prostatectomy should be withheld in the pandemic setting with restricted access to postoperative care and management of complications.18 Hormonal therapy using luteinizing hormone releasing hormone (LHRH) agents or surgical castration can be offered to anxious men, irrespective of the COVID-19 status, with delayed radical prostatectomy once resources are available.18,32 However, patients should be thoroughly explained about the low level evidence on neoadjuvant hormonal therapy and its uncertain benefits and surgery as part of MMT should be offered as and when resources are available.
Metastatic prostate cancer
Patients diagnosed to be metastatic during the pandemic should be started on hormonal therapy (six-monthly LHRH agents). Alternatively, surgical castration is an option in the pandemic setting.18,32 Prostate cancer patients on docetaxel chemotherapy are at high risk of neutropenia which, in turn, places them at high risk of COVID-19 systemic illness compared to the general population. Therefore, treatment with docetaxel can be deferred at least for 12 weeks considering the risk of side effects which enhance COVID-19 infections [Figure 3].18
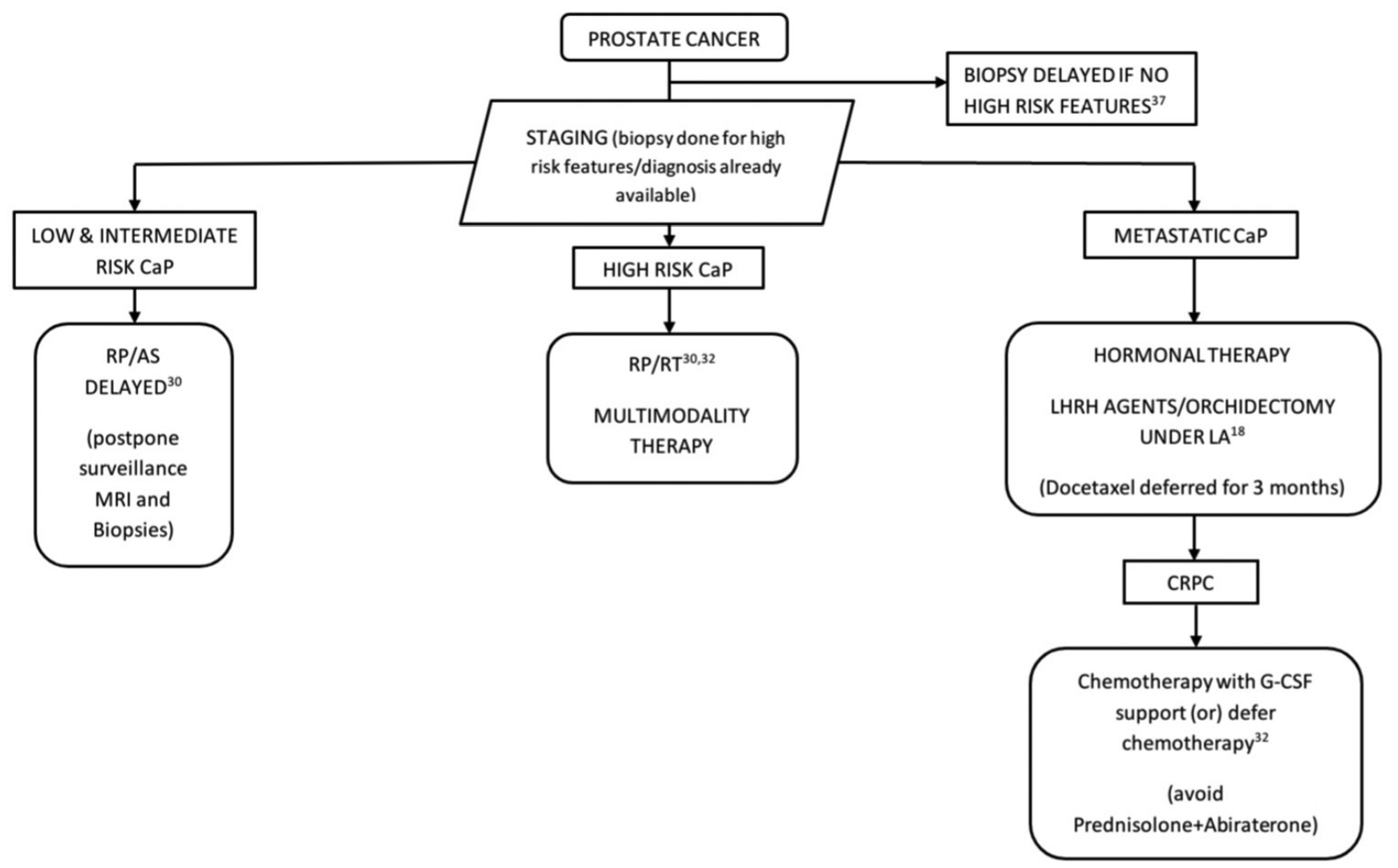
Figure 3: Algorithm for the management of prostate cancer in the COVID-19 pandemic. RP = Radical prostatectomy, LA = Local anesthesia, RT = Radiotherapy, LHRH agents = Luteinizing hormone releasing hormone agonist/antagonist, CaP = Cancer prostate, CRPC = Castration resistant prostate cancer, G-CSF = Granulocyte colony-stimulating factor
Patients on follow-up after radiotherapy Telemedicine consultations are preferred to avoid hospital visits. PSA levels need to be checked at 3 and 6 months after radiotherapy.18,32
Patients on follow-up after radical prostatectomy Serum levels of PSA can be tested 6–8weeks after surgery and followed up through telecommunication. If PSA <0.2 ng/ml, repeat testing is advised or deferred for 3 months. If PSA levels are >0.2 ng/ml patients can be offered two options; hormonal therapy followed by salvage radiotherapy once it is available or repeat PSA every 3 months and plan for PSMA PET scan when PSA rises above 0.5 ng/ml.18
Castration resistant prostate cancer
Hormonal management is preferred as chemotherapy is associated with significant immunosuppression, increasing the risk of COVID-19 infection. Abiraterone is avoided as the concomitant use of prednisolone is bothersome in case of COVID-19.32 If chemotherapy is planned, precautions such as combining with growth factors granulocyte-colony stimulating factor (G-CSF), reducing the number of cycles should be undertaken during the pandemic.32
Penile cancer
Diagnosis
The initial clinical visit can be replaced by a telemedicine consultation or clinical images shared through E-mail by the patient.18
Treatment
Procedures for the primary lesion such as glansectomy, gland resurfacing, circumcision, and partial penectomy can be performed under local anesthesia as a day care procedure which reduces the burden on hospitals.32 Topical chemotherapy or surveillance should be advised for in situ cancer.18 While a 3 month delay in the management of invasive penile cancers reduces the chances of future conservative management and sexual function, the impact on 5-year survival is insignificant. However, a delay beyond 6 months is associated with poorer survival.41 Delayed management of inguinal nodes by more than 3 months is associated with reduced 5-year survival and hence inguinal nodal dissection can be delayed up to 3 months or performed as a staged procedure.42 The management of pelvic nodal disease and metastatic penile cancer should be done on a case to case basis after discussing potential side effects and immunosuppression during the pandemic [Figure 4].
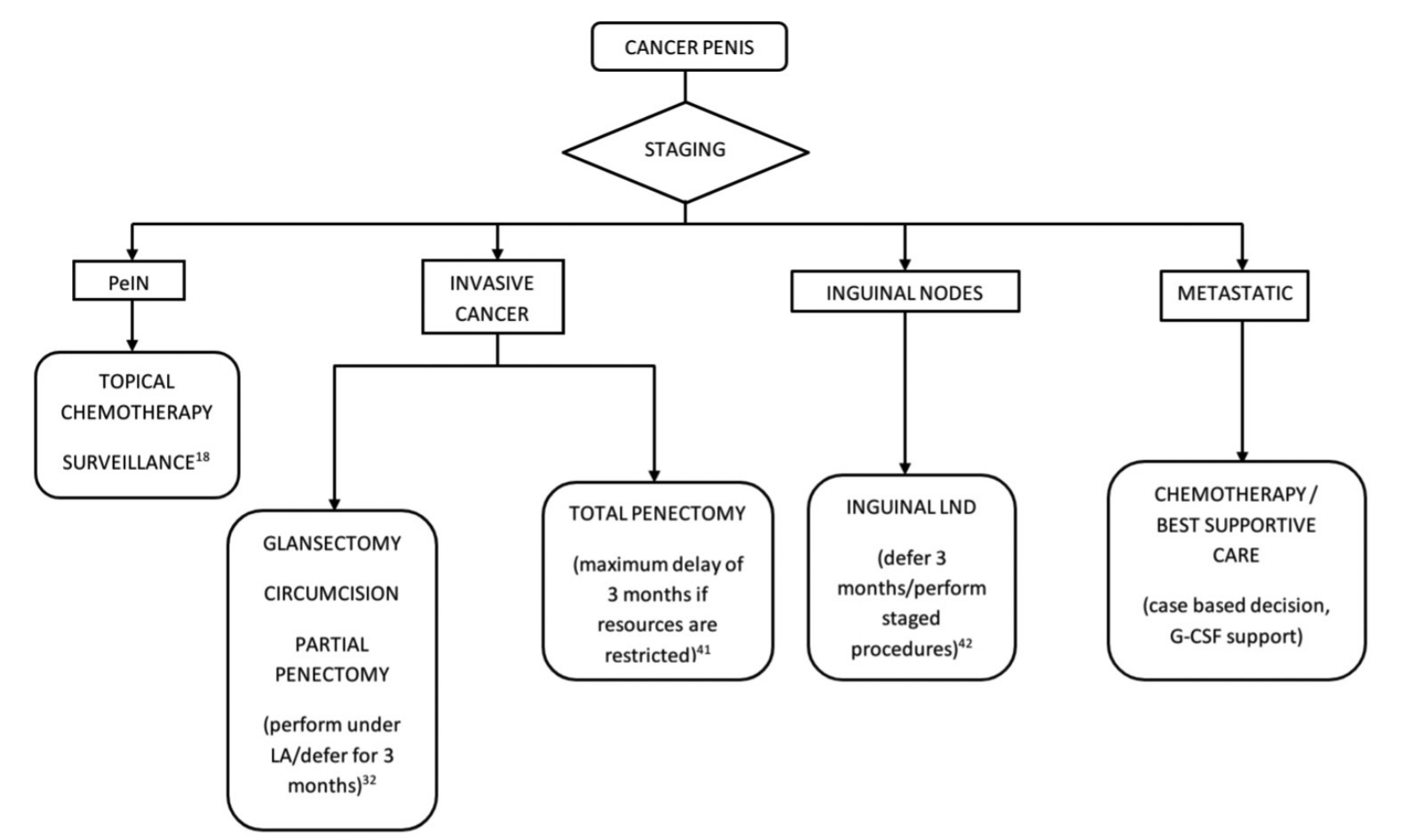
Figure 4: Algorithm for the management of penile cancer in the COVID-19 pandemic. PeIN-Penile intraepithelial neoplasia, LA = Local anesthesia, LND = Lymph node dissection
Testicular cancer
Diagnosis
Orchidectomy as a day care procedure should be performed because of the low level of evidence on delayed orchidectomy and its impact on survival.43 It is a procedure that confers survival benefit to the patient, and hence, must be prioritized during the pandemic.
Treatment
Surveillance must be advised whenever possible based on risk categorization. Adjuvant chemotherapy or radiotherapy after orchidectomy should be started during the pandemic when justified based on the risk factors.32 The chemotherapy regimen should be tailored with regards to concern for immunosuppression and negative effects on COVID-19. In this context, reducing the number of cycles, and G-CSF support are preferred. Bleomycin based regimens are better avoided considering the pulmonary toxicity and its possible deleterious effects on COVID-19 pneumonia. Ifosfamide is an effective alternative in this setting.32,44
Post chemotherapy retroperitoneal lymph node dissection is avoided considering the protracted and resource consuming postoperative care and the need for mechanical ventilators. Chemotherapy is an option, provided patients understand the risk of immunosuppression.45 First and second line chemotherapy for metastatic disease should not be stopped during the pandemic. However, G-CSF support and reduced number of cycles can help patients tide over this period and standard follow-up practices should be adhered to based on local availability [Figure 5].45
Conclusion
This review has discussed the salient practice modifications for efficient management of urological malignancies in the COVID-19 pandemic. However, it must be realized that the successful management of urological malignancies involves teamwork and co-operation between different cadres of the health care system. This review may not be complete due to the rapidly changing scenario in the present pandemic. Hence, local and hospital guidelines should dictate the management until more validated data about the viral infection in patients with genitourinary malignancies are available. It has to be emphasized that proper triage of cancer patients and their safe management is the need of the hour in these desperate times.
References:
- Cyranoski D. Mystery deepens over animal source of coronavirus. Nature 2020;579:18-9.
- WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19 – 11 March 2020. Available from: https://www.who.int/dg/ speeches/detail/who-director-general-s-opening-remarks-at-the-mediabriefing-on-covid-19---11-march-2020. [Last accessed on 2020 Apr 06].
- Wang LS, Wang YR, Ye DW, Liu QQ. A review of the 2019 novel coronavirus (COVID-19) based on current evidence. Int J Antimicrob Agents 2020;19:105948.
- Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis 2020. pii: ciaa272.
- Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention; 2020. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/index. html. [Last accessed on 2020 Apr 06].
- Chan MC, Yeo SE, Chong YL, Lee YM. Stepping forward: Urologists’ efforts during the COVID-19 outbreak in Singapore. Eur Urol 2020; Mar 17. Available from: https://www.europeanurology.com/article/S0302-28 38(20)30145-7/abstract. [Last accessed on 2020 Apr 01].
- Schoeman D, Fielding BC. Coronavirus envelope protein: Current knowledge. Virol J 2019;16:69.
- Pal R, Bhansali A. COVID-19, diabetes mellitus and ACE2: The conundrum. Diabetes Res Clin Pract 2020;162:108132.
- Cyranoski D. Did pangolins spread the China coronavirus to people? Nature 2020;Feb 7. Available from: https://www.nature.com/articles/ d41586-020-00364-2. [Last accessed on 2020 Apr 06].
- Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther 2020;14:58-60.
- Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 2020. pii: ciaa237.
- Baden LR, Rubin EJ. Covid-19 – The search for effective therapy. N Engl J Med 2020;382:1851-2.
- Mitjà O, Clotet B. Use of antiviral drugs to reduce COVID-19 transmission. Lancet Glob Health 2020;8:e639-40.
- Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, et al. Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Ann Oncol 2020. pii: S0923-7534(20)36383-3.
- Liang W, Guan W, Chen R, Wang W, Li J, Xu K. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol 2020;21:335-7.
- Wang H, Zhang L. Risk of COVID-19 for patients with cancer. Lancet Oncol 2020;21:e181.
- Lancet T. Palliative care and the COVID-19 pandemic. Lancet 2020;395:1168.
- Coronavirus & COVID-19. The British Association of Urological Surgeons Limited. Available from: https://www.baus.org.uk/about/ coronavirus_covid-19.aspx. [Last accessed on 2020 Apr 03].
- Aminian A, Safari S, Razeghian-Jahromi A, Ghorbani M, Delaney CP. COVID-19 outbreak and surgical practice: Unexpected fatality in perioperative period. Ann Surg 2020. pii: 10.1097/ SLA.0000000000003925.
- Kutikov A, Weinberg DS, Edelman MJ, Horwitz EM, Uzzo RG, Fisher RI. War on Two fronts: Cancer care in the time of COVID-19. Ann Intern Med 2020;Mar 27; . Available from: https://annals.org/ aim/fullarticle/2764022/war-two-fronts-cancer-care-time-covid-19. [Last accessed on 2020 Apr 01].
- Cancer Care during the Spread of Coronavirus Disease 2019 (COVID-19) in Italy: Young Oncologists’ Perspective. Esmo Open. Available from: https://esmoopen.bmj.com/content/5/2/e000759.long [Last accessed on 2020 Apr 03].
- Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020;368:m1295.
- Wang T, Chen R, Liu C, Liang W, Guan W, Tang R, et al. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol 2020;7:e362-3.
- Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost 2020;18:1023-6.
- Telehealth: What Urologists Need to Know – American Urological Association. Available from: https://www.auanet.org/advocacy/ what-urologists-need-to-know-about-telehealth. [Last accessed on 2020 Apr 03].
- TSI. Home. Available from: https://tsi.org.in/. [Last accessed on 2020 Apr 08].
- Mehrazin R, Smaldone MC, Kutikov A, Li T, Tomaszewski JJ, Canter DJ, et al. Growth kinetics and short-term outcomes of cT1b and cT2 renal masses under active surveillance. J Urol 2014;192:659-64.
- McIntosh AG, Ristau BT, Ruth K, Jennings R, Ross E, Smaldone MC, et al. Active surveillance for localized renal masses: Tumor growth, delayed intervention rates, and 5-yr clinical outcomes. Eur Urol 2018;74:157-64.
- Lardas M, Stewart F, Scrimgeour D, Hofmann F, Marconi L, Dabestani S, et al. Systematic review of surgical management of nonmetastatic renal cell carcinoma with vena caval thrombus. Eur Urol 2016;70:265-80.
- Ficarra V, Novara G, Abrate A, Bartoletti R, Crestani A, De Nunzio C, et al. Urology practice during COVID-19 pandemic. Minerva Urol Nefrol 2020;Mar 23.
- Bersanelli M. Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy 2020;12:269-73.
- Méjean A, Rouprêt M, Rozet F, Bensalah K, Murez T, Game X, et al. Recommendations CCAFU on the management of cancers of the urogenital system during an epidemic with Coronavirus COVID-19. Prog Urol 2020;30:221-31.
- Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARSCoV-2 in Different Types of Clinical Specimens. JAMA 2020;323:1843-4.
- Ling Y, Xu SB, Lin YX, Tian D, Zhu ZQ, Dai FH, et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl) 2020;133:1039-43.
- Hegarty P, Kamat A, Zafirakis H, Dinardo A. BCG Vaccination May be Protective Against Covid-19; 2020.
- Taniguchi K, Koga S, Nishikido M, Yamashita S, Sakuragi T, Kanetake H, et al. Systemic immune response after intravesical instillation of bacille Calmette–Guérin (BCG) for superficial bladder cancer. Clin Exp Immunol 1999;115:131-5.
- Katz EG, Stensland KS, Mandeville JA, MacLachlan LS, Moinzadeh A, Sorcini A, et al. Triaging office-based urology procedures during the COVID-19 pandemic. J Urol 2020. pii: 101097JU0000000000001034.
- Liu Y, Lu J, Huang Y, Ma L. Clinical spectrum of complications induced by intravesical immunotherapy of bacillus Calmette-Guérin for bladder cancer. Oncol 2019;2019:6230409.
- Bourgade V, Drouin SJ, Yates DR, Parra J, Bitker MO, Cussenot O, et al. Impact of the length of time between diagnosis and surgical removal of urologic neoplasms on survival. World J Urol 2014;32:475-9.
- Skouteris VM, Crawford ED, Mouraviev V, Arangua P, Metsinis MP, Skouteris M, et al. Transrectal ultrasound – Guided versus transperineal mapping prostate biopsy: Complication comparison. Rev Urol 2018;20:19.
- Gao W, Song L, Yang J, Song N, Wu X, Song N, et al. Risk factors and negative consequences of patient’s delay for penile carcinoma. World J Surg Oncol 2016;14:124.
- Chipollini J, Tang DH, Gilbert SM, Poch MA, Pow-Sang JM, Sexton WJ, et al. Delay to inguinal lymph node dissection greater than 3 months predicts poorer recurrence-free survival for patients with penile cancer. J Urol 2017;198:1346-52.
- Huyghe E, Muller A, Mieusset R, Bujan L, Bachaud JM, Chevreau C, et al. Impact of diagnostic delay in testis cancer: Results of a large population-based study. Eur Urol. 2007;52:1710-6.
- O’Sullivan JM, Huddart RA, Norman AR, Nicholls J, Dearnaley DP, Horwich A. Predicting the risk of bleomycin lung toxicity in patients with germ-cell tumours. Ann Oncol 2003;14:91-6.
- COVID-19 Resource: European Urology. Available from: https://www. europeanurology.com/covid-19-resource. [Last accessed on 2020 Apr 04].
- Department of Urology, Pondicherry Institute of Medical Sciences, Puducherry, Department of Urology, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India


