To date, the underlying pathophysiological mechanisms are of bone loss and higher fracture risk in stone formers have not been fully elucidated. In most studies hypercalciuria5 which is the most important urinary biochemical abnormality detected in calcium stone formers was perceived as the most important risk factors in the pathogenesis for bone loss in this population. Due to the aforementioned circumstances, we explored the association between urine calcium excretion and bone mineral density (BMD) in a large cohort of patients with nephrolithiasis. To overcome the limitations of the previous studies, in this population we included kidney stone formers from both genders, on a random diet and following restricted salt intake, and in the fasting condition to examine the physiologic conditions of renal calcium excretion.
In our recent study, for the first time after following adjustments for multiple factors which influence urinary calcium excretion, we detected no significant association between urinary calcium and BMD at the vertebral spine or femoral neck in estrogen repleted women and in males. In contrast, we found a significant negative relationship between urinary calcium and BMD at the vertebral spine in none-estrogen treated postmenopausal women (figure1a and 1b). We further showed that this inverse association was amplified with dietary calcium intake restriction. The significant inverse relationship urinary calcium and BMD in vertebral spine in non-estrogen treated women was anticipated, given that estrogen lack mainly influences vertebral spine. This result indicates that increased bone resorption is responsible for this finding in estrogen depleted postmenopausal women.
Osteoporosis is a disorder comprised of imbalance in bone formation and bone resorption, and limited bone biopsy studies performed in kidney stone formers have showed decreased bone formation as a hallmark in this population6,7. From these findings, one may argue that hypercalciuria solely could not be pathogenic in inducing bone loss and maybe only a surrogate in this population. One potential underlying factor maybe increased serum calcitriol levels or increase sensitivity to this metabolite, influencing target organs differently. The derangements at one hand increased intestinal calcium absorption but on the other hand has inhibitory roles on bone formation8-10 . There are similarities between underlying pathophysiologic mechanisms of bone loss in kidney stone formers and idiopathic osteoporotic men who showed an impaired bone formation concurrently with elevated calcitriol levels and elevated urinary calcium excretion11.
The future directions must be targeted further investigating underlying pathophysiologic mechanism of impaired bone formation in this population. Based on current knowledge it is suitable to consider BMD analysis to be performed in all kidney stone formers. Two major hurdles are insurance constraints in the United States making the BMD measurements and bone turnover markers in this population not reimbursable. Therefore, we hope that further awareness of practicing physicians and clinical investigators in the field will make the governmental agencies and National Institute of Health persuaded to facilitate reimbursement and further research in this population.
Based on present studies, an optimal therapeutic regimene that enhances bone formation will would be the ideal and be superior to bisphosphonates, which are known to affect bone turnover and further diminish bone formation, culminating in poor bone quality and fractures. Since Tthe only bone-anabolic agent available in the United States is teriparatide, a parathyroid hormone (PTH) analog, and itswhich use of is ofa concern in the kidney stone forming population due to its potential hypercalciuric effect. The, the combined use of thiazide diuretics combined with alkali treatment is a potential regimen for the stone forming population. The lLimited studies available of the use of combined thiazide and alkali treatment showing persistent increaseds in BMD in this population, suggesting that the effect of these agents on the bone may supersede go beyond their hypercalciuric effect and may potentially could be due to stimulation of bone formation2.
At the present time, the effect of currently available medications for thein treatment of kidney stone disease on bone marrow density has been limited to a few short term, nonrandomized, placebo-controlled studies. Given the high frequencyimpact of kidney stones and accompanied by bone fracture, larger multicenter studies are absolutely neededvital to further explore further the impact of such treatment in this population.
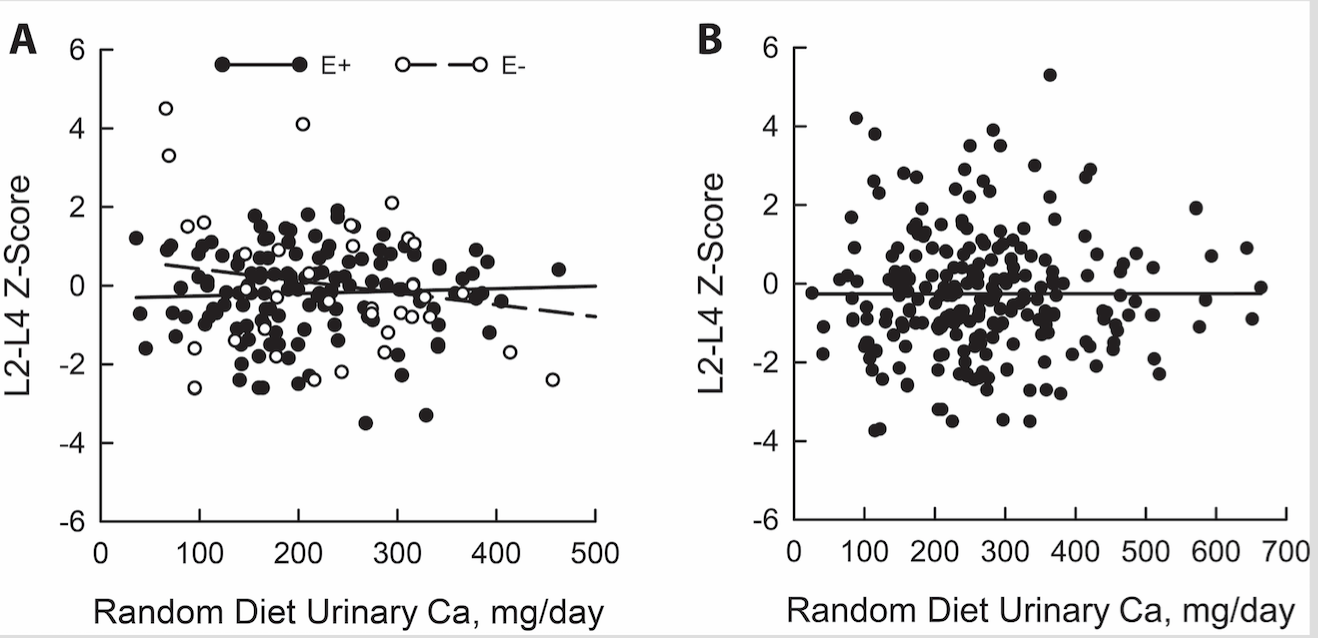
Legend to figures:
Figure 1A Relationship between urinary calcium and L2 and L4 spine BMD in estrogen treated (E2+) and untreated (E2-) women with kidney stones.
(With permission J. Urol., 2017)
Figure 1B Relationship between urinary and L2 and L4 spine BMD in men with kidney stones.
(With permission J. Urol., 2017)
Written by: Khashayar Sakhaee M.D.; Naim M. Maalouf M.D.; John Poindexter B.S.; Beverly Adams-Huet M.S.; Orson W. Moe M.D.
Department of Internal Medicine, Division of Mineral Metabolism and Charles, and Jane Pak Center for Mineral Metabolism and clinical Research
The University of Texas Southwestern Medical Center at Dallas, Dallas TX
Corresponding Author:
Khashayar Sakhaee, M.D., University of Texas Southwestern Medical Center, The Charles and Jane Pak Center for Mineral Metabolism and Clinical Research
Acknowledgement given to Ruby Aguire for preparation of this manuscript
References:
1. Pearle MS, Calhoun EA, Curhan GC. Urologic diseases in America project: urolithiasis. J Urol 2005;173:848-57.
2. Sakhaee K, Maalouf NM, Kumar R, Pasch A, Moe OW. Nephrolithiasis-associated bone disease: pathogenesis and treatment options. Kidney Int 2011;79:393-403.
3. Melton LJ, 3rd, Crowson CS, Khosla S, Wilson DM, O'Fallon WM. Fracture risk among patients with urolithiasis: a population-based cohort study. Kidney Int 1998;53:459-64.
4. Carbone LD, Hovey KM, Andrews CA, et al. Urinary Tract Stones and Osteoporosis: Findings From the Women's Health Initiative. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2015;30:2096-102.
5. Coe FL, Favus MJ, Crockett T, et al. Effects of low-calcium diet on urine calcium excretion, parathyroid function and serum 1,25(OH)2D3 levels in patients with idiopathic hypercalciuria and in normal subjects. The American journal of medicine 1982;72:25-32.
6. Bordier P, Ryckewart A, Gueris J, Rasmussen H. On the pathogenesis of so-called idiopathic hypercalciuria. The American journal of medicine 1977;63:398-409.
7. Heller HJ, Zerwekh JE, Gottschalk FA, Pak CY. Reduced bone formation and relatively increased bone resorption in absorptive hypercalciuria. Kidney Int 2007;71:808-15.
8. Breslau NA, Preminger GM, Adams BV, Otey J, Pak CY. Use of ketoconazole to probe the pathogenetic importance of 1,25-dihydroxyvitamin D in absorptive hypercalciuria. The Journal of clinical endocrinology and metabolism 1992;75:1446-52.
9. Zerwekh JE, Pak CY, Kaplan RA, et al. Pathogenetic role of 1 alpha,25-dihydroxyvitamin D in sarcoidosis and absorptive hypercalciuria: different response to prednisolone therapy. The Journal of clinical endocrinology and metabolism 1980;51:381-6.
10. Raisz LG, Kream BE, Smith MD, Simmons HA. Comparison of the effects of vitamin D metabolites on collagen synthesis and resportion of fetal rat bone in organ culture. Calcif Tissue Int 1980;32:135-8.
11. Zerwekh JE, Sakhaee K, Breslau NA, Gottschalk F, Pak CY. Impaired bone formation in male idiopathic osteoporosis: further reduction in the presence of concomitant hypercalciuria. Osteoporos Int 1992;2:128-34.
Read the Abstract


