Testosterone deficiency (TD) contributes to a host of pathophysiological processes and affects men’s overall health and quality of life.2,12,13 TD adversely reduces bone mineral density and muscle mass and increases fat mass contributing to larger body mass index (BMI). TD contributes to anemia, frailty, fatigue, and insulin resistance (IR). Other adverse effects of TD include altered mood, diminished vitality, and reduced level of energy and sense of well-being coupled with impaired memory and reduced cognition. TD is also associated with reduced libido, increased erectile, and orgasmic dysfunction. TD correlates with poor physical and social function and decline in overall health.2,12,13 TD predicts metabolic syndrome (MetS), diabetes, and obesity.2,12,13
Since MetS, obesity, and diabetes are risk factors for cardiovascular disease (CVD), it is likely that TD increases CVD risk as a result of potentiating such risk factors. Antonio et al and Laaksonen et al14,15 have shown that reduced T levels are independent predictors of MetS. Furthermore, in a large, well-executed epidemiological study with a long follow-up period, it was demonstrated that higher endogenous T levels are protective and associated with a reduced risk of CVD, whereas reduced T levels are associated with an increased risk of cardiovascular (CV) events, coronary heart disease, and cerebrovascular (CBV) disease.16
Recent reviews12,13 suggested that T therapy (TTh) in men with TD is not associated with increased CV risk. On the contrary, TTh appears to be protective. It should be pointed out that TTh has been used for over 70 years16-22 with little or no demonstrable risk. In fact, recent studies suggested that TTh does not increase CV risk or mortality and is thought to be beneficial.23-30 Of 9 meta-analyses published to date, all but 1 demonstrated that no serious harm is incurred from TTh; on the contrary, TTh is associated with significant overall health benefits.12,13 It is important to point out that since obesity, diabetes, IR, dyslipidemia, MetS, hypertension, and hyperglycemia are considered CV risk factors, any therapeutic modality that ameliorates these components is expected to reduce CV risk. Thus, it is not surprising that as published reports demonstrate that TTh ameliorates MetS; improves lipid profile, hyperglycemia, blood pressure, inflammation, and IR; increases lean body mass; improves bone mineral density; reduces waist circumference (WC); and improves vigor and vitality, TTh is also likely to reduce the risk of CVD and mortality.12,13
Over the past several years, 4 reports appeared in the clinical literature purporting increased CV risk and death attributed to TTh.31-34 A thorough analysis of these studies has been undertaken by several investigators12,13 as well as the Food and Drug Administration (FDA),35 all arriving at the conclusion that such studies are neither credible nor convincing with regard to the purported CV risk, due to methodological flaws, data contamination, and use of unvalidated statistical methods. Seven recent studies23-30 did not confirm the findings of these purported studies.31-34 On the contrary, none reported an association with TTh and increased CV risk or increased mortality. A recent randomized controlled trial of 790 men treated or untreated with T for 1 year confirmed no increase in the risk of CVD.30 On the contrary, in the second year of follow-up, the study showed more CV events in the placebo arm than in the T-treated group.30
We have undertaken this study to investigate the risks and benefits of TTh in men with TD treated for up to 8 years and compare these benefits with those in men with TD who remained untreated for the same length of time in a clinical setting that represents what is observed in real life. Our findings are summarized in this report.
Patients and Methods
This was an observational, prospective, cumulative registry study in 656 men (age: 60.72 + 7.15 years) with total T levels 12.1 nmol/L and symptoms of hypogonadism. Ethical guidelines as formulated by the German “A ̈ rztekammer” (the German Medical Association) for observational studies in patients receiving standard treatment were followed. After receiving an explanation regarding the nature and the purpose of the study, all participants consented to be included in the registry and have their data analyzed. Measurements of the parameters assessed in this study were carried out as previously described.36,37
Men seeking medical treatment for urological complaints were enrolled. In the T-treated group, 360 men received parenteral T undecanoate (TU) 1000 mg/12 weeks following an initial 6-week interval for up to 10 years. Men (n 1⁄4 296) who had opted against TTh, primarily due to financial reasons but also due to a negative perception of TTh as a risky treatment, served as controls. Median follow-up in both groups was 7 years.
Assessment and Follow-Up
Measurements were taken at least twice a year, and 8-year data were analyzed. We measured or calculated the following parameters—total plasma T levels, weight, WC, BMI, hemoglobin, hematocrit, fasting glucose levels and glycated hemoglobin (HbA1c), systolic blood pressure (SBP) and diastolic blood pressure (DBP), heart rate, pulse pressure, rate pressure product, lipid profile (total cholesterol [TC], low-density lipoprotein [LDL]-cholesterol, high-density lipoprotein [HDL]-cholesterol, triglycerides [TGs]), C-reactive protein, and liver transaminases. We also assessed prostate volume and prostate-specific antigen and questionnaires including the International Prostate Symptom Score (IPSS), Aging Males’ Symptoms (AMS), and International Index of Erectile Function, Erectile Function domain (IIEF-EF). Measures were taken between 2 and 4 times per year and annual average was calculated.
Statistical Methods
In the treated group, patients returned quarterly for TU injections, whereas in the control group, patients returned biannually for a routine visit. Data in both treated groups have been averaged across each year of patients participating in the study. Thus, obtained yearly data were used to assess differences between the 2 groups while adjusting for possible confounding. Adjusted multivariable analyses and the propensity score matching approaches were used to compare the 2 groups across time while adjusting for baseline differences.
Adjusted Multivariable Analyses
In adjusted multivariable analyses, changes from baseline in parameters (weight, WC, etc) were analyzed using a mixed model for repeated measures in terms of treatment, visit, and treatment-by-visit interaction as fixed factors and age, WC, weight, systolic and DBP, TC, HDL, LDL, TG, AMS, glucose, and baseline values of the analysis parameter as covariates. Baseline parameter values are the values recorded prior to TU injection. A random effect was included in the model for the intercept. Adjusted mean differences between treatment groups at each time point and across time within each treatment group were estimated using estimate statements in SAS PROC MIXED, Version 9.3 (2011) provided by SAS Institute Inc, Cary, North Carolina.
Propensity Matching Analyses
Our general strategy for propensity matching of those on active treatment to those who remained untreated included calculating propensity score based on logistic regression model and selecting matching pairs (or one to many) based on the score. The matching was performed by “nearest neighbor” selection with caliper set to a fraction of standard deviation (SD) of the propensity score. Several scenarios were considered. We first attempted to create propensity score based on following variables—age, WC, weight, SBP and DBP, TC, HDL, LDL, TG, AMS, and glucose. That model discriminated between active drug and those who remained untreated too well, resulting in a very small overlap of propensity score distributions. We then created propensity score based on the following variables— age, BMI, and WC. The 1:1 matching was done choosing nearest neighbor match with caliper set to 0.2 SD of the propensity score. Additionally, we explored 1:1 matching setting caliper to 0.5 SD and 1:2 matching with 0.2 SD and 0.5 SD calipers. These additional scenarios did not result in noticeable gain of the matched sample. Analyses were performed using SAS 9.3 software (SAS Institute, Cary, North Carolina).
Results
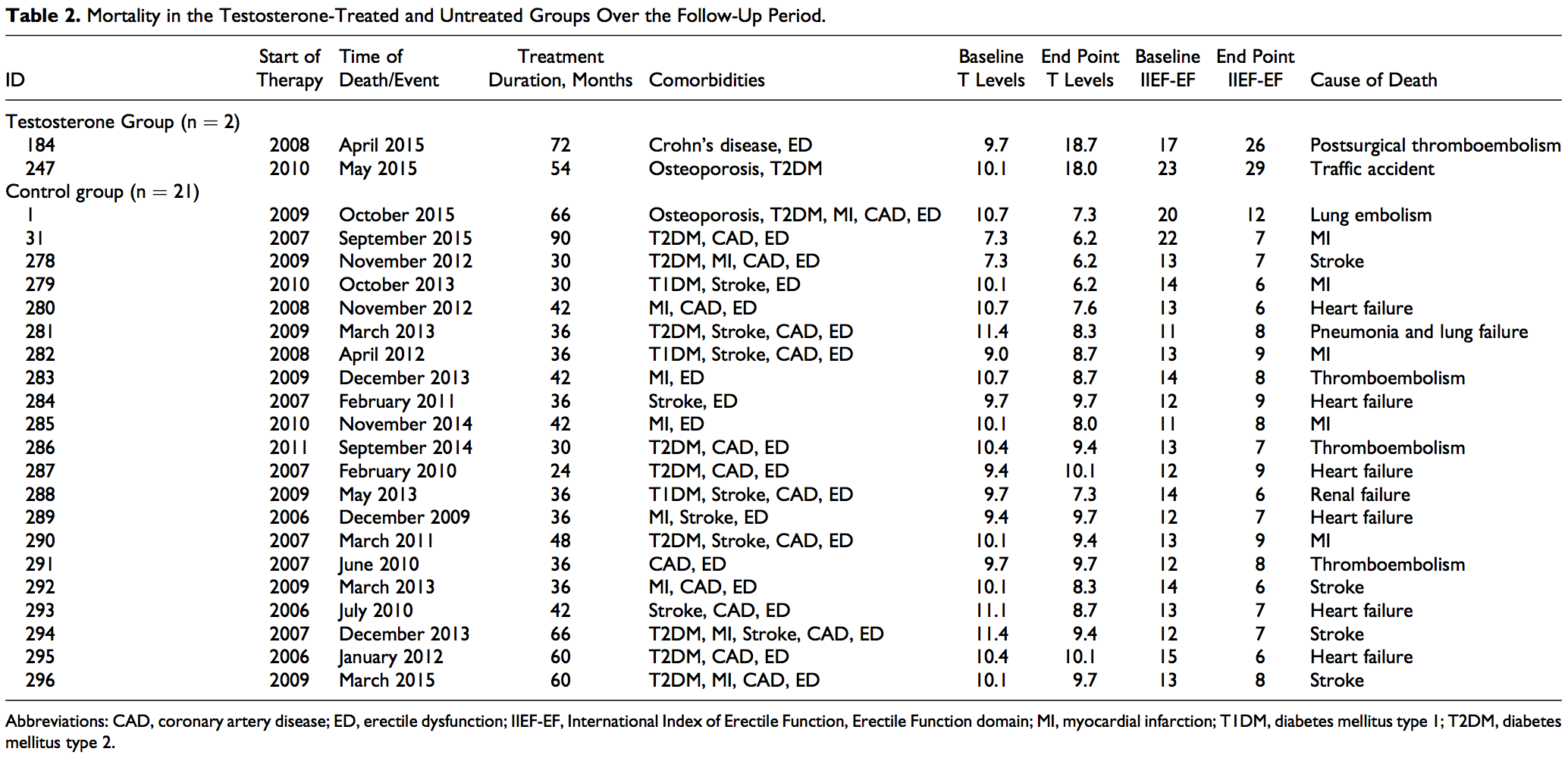
Baseline characteristics and comorbidities of the patients included in this registry and reported in this article are shown in Table 1. A total of 656 patients were included in the study and were followed up for up to 8 years. In the group that opted against TTh (henceforth referred to as untreated, control group), a total of 296 patients were followed up. The mean baseline age was 64.8 + 4.3 years, with a mean follow-up of 6.5 + 1.2 years and a median follow-up of 7 years. The T-treated group consists of a total of 360 patients with a mean baseline age of 57.4 + 7.3 years, with a mean follow-up of 6.5 + 2.4 years and a median follow-up of 7 years. In the control group, there were 12 men who were diagnosed with prostate cancer during the follow-up period. In the T-group, there were 7 men who were diagnosed with prostate cancer during the follow-up period. Furthermore, in the control group, there were 21 deaths, 19 of which were attributed to CVD. In the T-group, 2 deaths occurred, none was attributed to CVD. We should emphasize that the 2 groups are compared in terms of changes from baseline rather than the absolute values. This was done, in part, to ensure that differences between the 2 groups at baseline do not contribute to the observed differences between the groups. The data presented here reflect the estimated adjusted mean difference between the 2 groups.
Impact of TTh on Mortality and Nonfatal Myocardial Infarction and Stroke
In this registry, the follow-up time for the total group (in months) was 73.29 + 22.9 (minimum: 9; maximum: 111) and in the control group was 74.37 + 13.60 (minimum: 24; maximum: 90). In the T-treated group, the follow-up time was 72.4 + 28.35 (minimum: 9; maximum: 111). As shown in Table 2, there were 2 deaths in the T-treated group, none was related to CV events. One was attributed to postsurgical thromboembolism and 1 due to traffic accident. In the nontreated control group, there were 21 deaths, 19 of which were related to CV events. Five were attributed to myocardial infarction (MI), 4 were attributed to stroke, 7 were attributed to heart failure, 2 to thromboembolism, 1 to lung embolism, and 1 to pneumonia and lung failure (Table 2). The incidence of death in 10 years was 0.1145 in the control group (95% confidence interval [CI]: 0.0746-0.1756; P < .000) and 0.0092 in the T-treated group (95% CI: 0.0023-0.0368; P < .000); the estimated difference between the groups was 0.0804 (95% CI: 0.0189-0.3431; P < .001). The estimated reduction in mortality for the T-group was between 66% and 92%. There were 26 nonfatal MIs (Table 3) and 30 nonfatal strokes (Table 4) in the control group and none in the T-treated group.
Impact of TTh on Hyperglycemia and HbA1c Levels in Men with Hypogonadism Treated or Untreated With TTh for up to 8 Years
TTh reduced blood glucose levels significantly in men with hypogonadism (5.7 + 0.7 to 5.2 + 0.1 mmol/L). When data were adjusted for baseline differences, the adjusted difference between the treated and untreated control groups showed a progressive decrease in glucose levels from baseline (Figure 1A). The estimated change from baseline was 0.4 mmol/L (P < .0001). In contrast, blood glucose levels in untreated men did not show demonstrable changes (5.6 + 0.4-5.6 + 0.3 mmol/L). The change from baseline was 0.002 mmol/L (not significant [NS]). The most profound observation is the noted change in HbA1c levels in men treated with T when compared to the untreated group (Tables 5 and 6). As shown in Figure 1B, HbA1c levels were significantly reduced in the T-group, and the reduced values were maintained with TTh over the course of follow-up. Glycated hemoglobin was recorded from 6.9% + 1.4% to 5.6% + 0.4%, with an estimated change from baseline of 1.7% (P < .0001). After adjustment for baseline differences, the adjusted difference between the treated and untreated control groups showed a progressive decrease in HbA1c from baseline (Figure 1B). In contrast, HbA1c increased in the untreated group from baseline 6.1% + 1.2% to 6.4% + 1.4%, with an estimated change from baseline of þ0.3% (P < .0001).
Subgroup analysis comparing the effects of TTh in diabetic men showed considerable and significant reductions in HbA1c values compared to diabetic men who remained untreated (control group; data not shown). This is consistent with observations reported previously by others.38-43 The reductions in HbA1c by TTh have important implications in reducing the IR burden in diabetic men and also in reducing the risk of CVD.


Impact of TTh on SBP and DBP in Men with Hypogonadism Treated or Untreated With TTh for up to 8 Years
Systolic blood pressure decreased from 151.3 + 17.0 mm Hg to 130.0 + 6.6 mm Hg in the T-group (P < .0001) and increased slightly but significantly from 139.5 + 15 mm Hg to 140.3 + 13.3 mm Hg in the control group (P < .0005). Diastolic blood pressure decreased from 90.6 + 11.6 mm Hg to 74.4 + 4.6 mm Hg in the T-group (P < .0001) and increased slightly but significantly from 79.6 + 9.2 mm Hg to 81.1 + 8.4 mm Hg in the control group (P < .005). After adjustment for baseline differences, the adjusted difference between the treated and untreated control groups showed a progressive decrease in SBP and DBP from baseline (Figure 2A and B). Pulse pressure, a marker of arterial stiffness, decreased in the T-group from 60.7 + 7.7 mmHg to 55.6 + 4.9 mmHg (P < .0001) and remained unchanged in the control group. Heart rate (beats per minute) decreased in the T-group from 77.5 + 3.7 to 72.4 + 2.1 (P < .0001) and increased slightly but significantly in the control group from 76.2 + 5.0 to 77.6 + 4.0 (P < .01). Rate pressure product decreased from 11 751 + 1610 to 9421 + 617 in the T-group (P < .0001) and increased from 10 623 + 1347 to 10 890 + 1106 in the control group (P < .0005), with an estimated difference between groups of 2656 (Tables 5 and 6). These findings suggest that long-term TTh in men with hypogonadism resulted in significant reductions in both SBP and DBP as reported previously.36,37,44,45-53

Impact of TTh on Lipid Profiles in Men with Hypogonadism Treated or Untreated With TTh for up to 8 Years
As shown in Figure 3A-D and Tables 5 and 6, TTh produced significant decrease in TC (mmol/L) from 7.2 + 1.1 to 4.8 + 0.2 (P < .0001), whereas in the control group, TC increased from 6.3 + 1.2 to 6.8 + 1.1 (P < .0001). After adjustment for baseline differences, the difference between the treated and untreated control groups showed a progressive decrease in TC from baseline (Figure 3A). In the control group, LDL increased from 3.5 + 1.5 to 4.0 + 1.5 (P < .0001) but was significantly reduced in the T-group. After adjustment for baseline differences, the difference between the treated and untreated control groups showed a progressive decrease in LDL-cholesterol from baseline (Figure 3B). TTh increased HDL levels (mmol/L) from 1.4 + 0.5 to 1.9 + 0.5 (P < .0001). We also noted an increase in the control group (untreated) from 1.3 + 0.5 to 1.6 + 0.7 (P < .0001). This increase in HDL levels in the T-group is accompanied by significant reductions in LDL levels (mmol/L) from 4.2 + 1.1 to 2.7 + 0.8 (P < .0001). After adjustment for baseline differences, the difference between the treated and untreated control groups showed a progressive increase in HDL-cholesterol from baseline (Figure 3C). Triglyceride levels (mmol/L) decreased in the T-group from 3.1 + 0.6 to 2.1 + 0.1 (P < .0001) and increased in the control group from 2.9 + 0.6 to 3.1 + 0.6 (P < .0001). After adjustment for baseline differences, the difference between the treated and untreated control groups showed a progressive decrease in TG levels from baseline (Figure 3D). Most importantly, the TC/HDL ratio was reduced in both groups but did not reach statistical significance in the untreated (control) group. As shown in Figure 4A, the difference between the treated and untreated groups showed a progressive decrease in the TC/HDL ratio from 5.6 + 1.9 to 2.6 + 0.7 in the T-group (P < .0001) and from 6.2 + 3.5 to 5.6 + 3.5 in the control group (NS). Since TC/HDL ratio is considered as an important parameter for CV risk assessment, this observation is of considerable significance to the role of TTh and CV risk. Finally, non-HDL cholesterol (mmol/L) decreased in the T-group from 5.8 + 0.9 to 2.8 + 0.5 (P < .0001) and increased in the control group from 5.0 + 1.3 to 5.2 + 1.4 (P < .0001). After adjustment for baseline differences, the difference between the treated and untreated control groups showed a progressive decrease in non-HDL cholesterol from baseline (Figure 4B).

Figure 1. Changes in fasting blood glucose and glycated Hemoglobin (HbA1C) in the testosterone (T)-treated and untreated (control) groups. A, Changes in glucose levels (yellow bars) were adjusted for baseline differences between the T-treated (green bars) and untreated (red bars) control groups. B, Changes in HbA1c (yellow bars) were adjusted for baseline differences between the T-treated (green bars) and untreated (red bars) control groups.
Impact of TTh on Liver Function Enzymes in Men with Hypogonadism Treated or Untreated With TTh for up to 8 Years
Testosterone therapy produced a gradual and progressive decrease in liver transaminases (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]), suggesting potential reduction in liver fat content and inflammatory activity. In contrast, an increase in liver transaminases is noted in the untreated (control) group. In the T-group, AST decreased from 39.6 + 15.8 to 16.1 + 2.4 U/L (P < .0001). In the control group, AST increased from 23.4 + 4.8 to 40.3 + 7.7 U/L (P < .0001). ALT decreased from 41.7 + 15.9 to 16.1 + 3.0 in the T group (P < 0.0001) and increased from 27.4 +4.9 to 44.4 +7.8 in the control group (P < .0001; Tables 5 and 6).
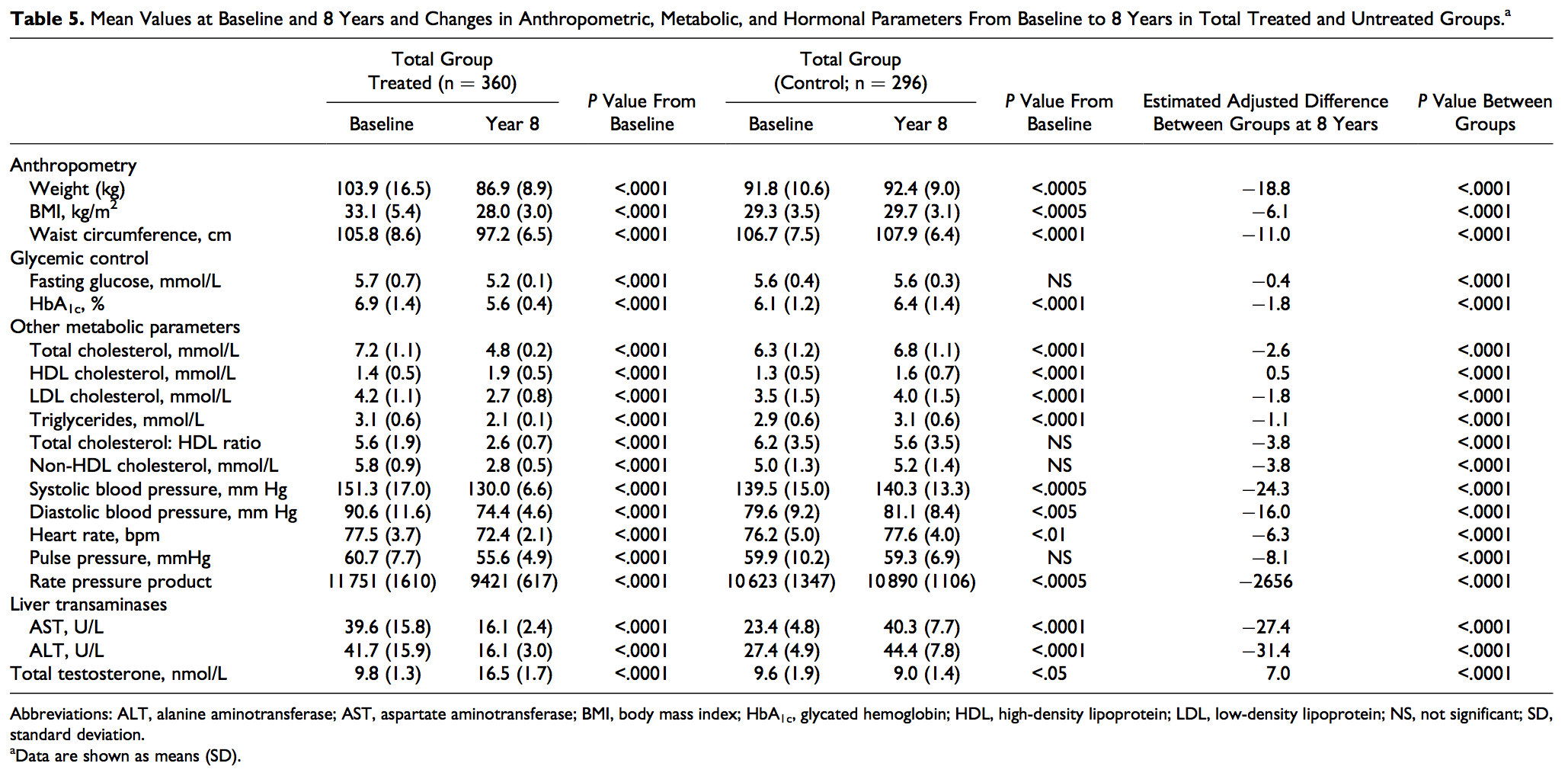


Figure 2. Changes in systolic and diastolic blood pressure in the testosterone (T)-treated and untreated (control) groups. A, Changes in systolic blood pressure (yellow bars) were adjusted for baseline differences between the T-treated (green bars) and untreated (red bars) control groups. B, Changes in diastolic blood pressure (yellow bars) were adjusted for baseline differences between the T-treated (green bars) and untreated (red bars) control groups.

Figure 3. Changes in lipid profile in the testosterone (T)-treated and untreated (control) groups. Changes in total cholesterol (A), low-density lipoprotein (LDL) cholesterol (B), high-density lipoprotein (HDL) cholesterol (C), and triglycerides (D; yellow bars) were adjusted for baseline differences between the T-treated (green bars) and untreated (red bars) control groups.

Figure 4. Changes in total cholesterol (TC)/high-density lipoprotein (HDL) ratio and non-HDL cholesterol in the testosterone-treated and untreated (control) groups. Changes in TC/HDL ratio (A) and non-HDL cholesterol (B; yellow bars) were adjusted for baseline differences between the T-treated (green bars) and untreated (red bars) control groups.
Impact of TTh on Anthropometric Parameters in Men with Hypogonadism Treated or Untreated With TTh for up to 8 Years
TTh in men with hypogonadism produced significant and sustained weight loss (WL) over the course of the treatment period (mean weight decreased from 103.9 + 16.5 kg to 86.9 + 8.9 kg); the changes in weight were statistically significant for all 8 years versus the previous year (P < .0001). The estimated mean change from baseline was 19.3 kg and the mean percent change from baseline 17.0% + 7.8%. In contrast, there was a slight but significant weight gain in the control group (mean weight increased from 91.8 + 10.6 kg to 92.4 + 9.0 kg; P < .0005). The estimated mean change from baseline was þ1.6 kg and the percent mean change from baseline þ1.5% + 2.4% (Tables 5 and 6). The WL noted in the T-group appears to translate into a marked reduction in WC. Waist circumference in the T-group decreased from 105.8 + 8.6 cm to 97.2 + 6.5 cm (P < .0001). The changes were statistically significant for 8 years versus the previous year (P < .0001). The estimated mean change from baseline was 10.0 cm. When the data were adjusted for baseline differences, the adjusted difference between the treated and untreated control groups showed a progressive decrease in WC from baseline (Figure 5). A slight increase in WC was observed in the untreated group. Waist circumference in this group increased from 106.7 + 7.5 cm to 107.9 + 6.4 cm (P < .0001). The observed WL and reduction in WC in the T-group are also reflected in reduced BMI values (BMI decreased from 33.1 + 5.4 to 28.0+3.0, estimated mean change from baseline 6.2 kg/m2). A slight but significant increase in BMI was noted in the untreated group where BMI increased from 29.3 + 3.5 to 29.7 + 3.1 by an estimated þ0.5 kg/m2 (P < .0005).

Figure 5. Changes in waist circumference (WC) in the testosterone (T)-treated and untreated (control) groups. Changes (yellow bars) were adjusted for baseline differences between the T-treated (green bars) and untreated (red bars) control groups.
Effects of Long-Term TTh on Safety Parameters in Men with Hypogonadism
In this comparison registry study, long-term TTh in men with hypogonadism increased hemoglobin concentrations and hematocrit, but the levels remained within the physiological ranges.36,37,44 Seven patients were diagnosed with low-grade prostate cancer in the T-group (1.9%) and 12 patients were diagnosed with prostate cancer in the untreated (control) group (4.1%).
Discussion
Epidemiological studies demonstrated that reduced circulating T levels are associated with greater CVD risk and physiological T levels are associated with a protective effect on the vascular system.16 However, to date, there are no published large, prospective, placebo-controlled studies of sufficient duration that investigated the effects of TTh, especially with regard to CVD, in men with hypogonadism and assessed the benefits and risks of TTh. A number of observational studies have demonstrated that TTh reduced mortality and produced improvements in CV risk factors, such as reduced fat mass, obesity, WC, blood pressure, and improvement in glycemic control.36,37,44
TD (hypogonadism), MetS, type 2 diabetes, and other known risk factors for CVD are chronic diseases requiring chronic, lifelong treatment. Indeed, assessment of TTh on these chronic conditions requires long-term randomized, controlled trials (RCTs) with long durations approaching a decade in order to truly assess what happens in real-life settings. Unfortunately, this is not feasible and most of the RCTs are of short duration. It is unlikely that we will be able to observe real-life changes in response to therapy in studies with short duration. Therefore, registry studies represent a bridge between RCTs and real life.54,55
In this report, we present data from an observational registry study on TTh in 360 men with hypogonadism who were followed up for a period of 8 years while on continuous TTh and compared these findings to data from 296 men with hypogonadism who remained untreated for the same follow-up period, approaching 8 years. Of particular interest is that there were only 2 deaths in the T-treated group and none was related to CV events. Interestingly, in the nontreated control group, there were 21 deaths, 19 of which were related to CV events. Furthermore, there were 26 nonfatal MIs and 30 nonfatal strokes in the control group but none in the T-treated group. These findings are in agreement with prior observational studies.24-30,56-58
TTh has been shown to reduce the risk of incidence of MI, stroke, and mortality in men with hypogonadism.24-30,56-58 These reports, together with the meta-analysis published by Corona et al59 and the FDA response to the petition to place a black box on T products,35 suggest that no credible or substantial evidence exists for increased CV risk with TTh. Our findings which span more than 8 years with a large number of patients also confirm this premise. Thus, we point out that the earlier reports that purported increased CV risk with TTh are confounded by methodological flaws and without adequate clinical acumen that makes them inconclusive, and at best suspect, in their conclusions. Considerable clinical benefits of TTh cannot be denied such as improvement in sexual desire and erectile function,59-62 increased energy, mood, and vitality,62-66 increased lean body mass67-71 reduction in total body fat mass,63-66,72-74 and reduction in WC.3,36,37,45,75
Importantly, TD in older men is associated with an increased risk of death.76,77 A number of studies demonstrated that TTh improves CV risk factors including reducing fat mass, ameliorating obesity, reducing WC, reducing blood pressure, and improving glycemic control.11,76-81 Similarly, the improvements in blood pressure, insulin sensitivity, HbA1c, and MetS components subsequent to TTh suggest that this therapeutic modality reduces CV risk.3,7,8,36,37,40,44,45,46,67-71,75,82-87
Epidemiological studies identified TD as a risk factor for CVD.88 Furthermore, TTh improves CBV perfusion and improves mood in men with TD and low T levels predict a poor CV risk profile.89,90 We should point out that we made no attempts to monitor changes in lifestyle, simply because when this registry study was initiated, there was no expectation that men would lose weight, lose WC, and experience improvement in lifestyle. For this reason, there were no plans to investigate the effects on changes in lifestyle, which is very important. However, placebo-controlled studies showed that obese men on a hypocaloric diet receiving T had a significant increase versus baseline in step count per day and activity, assessed by accelerometry.91 The patients in this registry also reported anecdotally that they had increased their level of physical activity. Future study should account for improvements in mood and quality of life in response to TTh.
We should also point out that several studies showed reduced carotid intima–medial thickness in response to TTh, suggesting that normalizing serum T may prevent or reverse atherosclerosis. In addition, TTh reduced mortality by approximately 50% in men with hypogonadism57 and diabetic men.58 A recent large observational study by Wallis et al92 demonstrated that in long-term TTh, an inverse relationship between TTh and CVD risk and mortality was observed. It is our view that such important findings provide support for the premise that TTh reduces mortality associated with CVD and TD increases mortality among men with hypogonadism.11,76-81
We also investigated the changes in blood glucose levels and the levels of the surrogate marker for hyperglycemia, HbA1c. Most importantly, we noted that TTh in men with hypogonadism resulted in significant and sustained reductions in blood glucose throughout the observation period. Interestingly, however, this was not the case in men with hypogonadism who remained untreated for the same observational period. The reduction in blood glucose may be explained by improved glucose uptake, utilization, and disposal in response to T action and in overall improvement in fuel metabolism. This finding is of importance, since hyperglycemia is a component of the MetS and a contributor to IR and onset of diabetes, thus contributing to increased CVD risk. The marked improvement in glucose metabolism resulting from TTh is also reflected in the reduction in the fraction of HbA1c. This observation is consistent with previous studies.36,37,42-44 We did not observe, however, any significant decrease in HbA1c levels in the untreated (control) group, confirming a role of T action in glucose utilization and disposal.93,94 This finding has relevant clinical implication for regulating hyperglycemia in men with hypogonadism. Since hyperglycemia, IR, and diabetes are considered as risk factors for CVD, therefore, TTh ameliorates hyperglycemia and IR and reduces the risk of CVD. Intensive glucoselowering therapy by various therapeutic modalities has been the mainstay of treating hyperglycemia. However, many of such therapeutic agents are associated with adverse side effects and poor compliance, and initial improvements cannot be maintained. T is a physiological hormone and, when administered in physiological levels, it produces marked reductions in glucose and HbA1c levels without serious side effects.36,37,42-44,63 Thus, this therapy may serve as a novel approach to augment treating hyperglycemia in men with hypogonadism. These findings further support the notion that TTh contributes to a reduction in CV risk and an improvement in cardiometabolic function.
One of the critical findings of this long-term study is the improvements and normalization of the lipid profile only in men with hypogonadism treated with T. Pronounced and significant decreases in TC, LDL, and TGs were observed in response to TTh over the course of the treatment period. In contrast, no significant changes were noted in the untreated (control) group. Since dyslipidemia is one of the components of the MetS and a risk factor for CVD, any normalization in the lipid profile would be considered a benefit since it reduces the risk of MetS and CVD. It is worth noting that the observed decreases in TC, LDL, and TGs in response to TTh are significant and parallel those values observed in men treated with statins to prevent CVD. More importantly, the TC/HDL ratio in the T-treated men was lowered significantly compared to untreated men. Since this ratio is thought to predict the risk of CVD, in particular, ischemic heart disease, such decreases in this ratio noted in this study support the notion that TTh reduces the risk of CVD.95
In this study, we also compare the changes in SBP and DBP in the T-group with that of the untreated group. Our findings showed a significant and gradual decrease in both SBP and DBP in patients treated with T but no significant decreases in blood pressures in the untreated patients (control group). The decrease in blood pressure in the T-group was maintained over the entire course of the 8 years of continuous therapy. The link between TD and risk of hypertension and the improvement in blood pressure with TTh has been proposed previously.44,96
Several studies have suggested that T modulates arterial blood pressure via a host of biochemical and physiological mechanisms,47,48 and low circulating T levels may contribute to hypertension. Systolic blood pressure is inversely associated with T levels,47,48 suggesting that hypogonadism contributes to higher blood pressure. Men with hypogonadism treated with TTh were shown to exhibit reduced blood pressure.47,48 Of interest is the improvement in pulse pressure, a surrogate marker for arterial stiffness, in the T-treated but not in the untreated group. It should be noted that pulse pressure is considered a marker of vascular stiffness and any reduction in this parameter is considered favorable for reducing CVD risk.97,98 This observation is congruent with data from a recent placebo-controlled study in which reduction in arterial stiffness was reported following TTh.99 The reduction in rate pressure product in the T-group reflects a decrease in the myocardial workload.
We further compared the effects of TTh on anthropometric parameters and risk factors relevant to cardiometabolic function, considered to be risk factors for CVD. The data presented here clearly demonstrate that TTh produces profound effects on the anthropometric parameters with concomitant WL, reduction in WC, and diminished BMI. No significant WL and reduction in WC and BMI were observed in the untreated group over the entire follow-up period. When findings obtained from the T-group were compared to the data obtained from the untreated (control) group, the changes in weight, WC, and BMI produced by TTh were significant and sustained over the entire treatment period. We believe that the observed WL and the reduction in WC and BMI are the results of TTh-inducing changes in body composition. It is well known that TTh invariably reduces fat mass and increases lean mass.3 This is critical in that increased lean body mass is thought to improve basal metabolic rate, glucose metabolism, overall health, and mortality.100,101 Thus, this suggests that TTh in men with hypogonadism may reduce the risk of CVD and provide a protective effect, as suggested by several contemporary studies.23-30,56
Although a considerable body of evidence accumulated to suggest that TTh does not increase the risk of CVD, a recent review by Huo et al102 tabulated studies on TTh in men with hypogonadism and suggested that studies that examined clinical CV end points have not favored TTh over placebo. It appears that since the purported risks of TTh regarding prostate cancer and CVD risk have been debunked, the authors attempted to downplay the benefits of TTh, especially with regard to the CV physiology. It should be pointed out that this review made a large tabulation of methods and end points of studies reported in the literature but failed to perform appropriate analyses, such as Forest plots or any other form of analyses to account for difference among studies in baseline characteristics, comorbidities, differing end points, varying degrees of clinical assessment, differing T formulations and route of administration, different durations of treatments, or adjusting for variables among the tabulated studies. Interestingly, the authors of this review102 formulated their own conclusions based not on actual data presented in such studies but rather on preconceived ideology. This review either ignored or overlooked the findings of many studies that demonstrated significant benefits of TTh.11-16,24-30,36-46,57-59,62-69
We wish to emphasize that in addition to the adjusted multivariable analyses used in this study, we have also utilized the propensity score matching approaches to compare the 2 groups across time while adjusting for baseline differences. The propensity matching analysis of men on active TTh with those untreated men, calculating propensity score based on logistic regression model and selecting matching pairs based on the score (see “Methods” section), was carried out to verify that the data obtained with the regression model were meaningful. We must point out that all additional analyses using various scenarios did not result in any noticeable gain of the matched sample and were congruent with data from the adjusted multivariable analysis model.
Study Limitations
The present study was not designed or powered to address the effects of TTh on mortality in men with hypogonadism. There was no adjudication of previous CV events that were reported by the patients as part of their anamnesis. Since patients were treated for their underlying diseases by other specialists than the urologist performing TTh, there was no precise monitoring of concomitant medications, so that changes cannot be excluded.
We do not have any information on medication adherence with regard to any of the concomitant medications that patients had been prescribed. Treatment decisions were made by the same single urologist (A.H.), and the same laboratory was used at all times. We wish to note that the majority of patients whether in the TTh group or in the control group were receiving the standard-of-care treatment in a limited number of general clinical practice or internist offices in and around the city of Bremerhaven, Germany. Thus, we believe that there were minimal variations in the overall management of these patients. For these reasons, it is unlikely that patients in one group received different treatment for their comorbidities from patients in the other group.
Another limitation is that patients were not randomized: The decision for or against TTh, however, was not possible for all patients. Patients with Klinefelter syndrome and other forms of primary hypogonadism had no choice and invariably received TTh, and so did patients with inflammatory bowel diseases who were specifically referred to be treated with T. The fact that these 3 subgroups were considerably younger explains the age gap between the T-group and the control group. We should also point out that potential selection bias may exist based on socioeconomic status—a factor well known to influence the overall health and CV health. Since patients opting not to receive TTh due to financial reasons are part of the control group, it is possible that patients who decided against T treatment for financial reasons did so because of their lower income.
Conclusion
In the absence of long-term prospective, placebo-controlled trials to investigate the risks and benefits of TTh in men with hypogonadism, observational registry studies that include a control group, such as reported herein, provide critical information on the long-term safety and effectiveness in clinical practice, especially relevant information regarding adherence and health outcomes in the general population.54,55,92,103,104 In contrast to the majority of studies, patients in the T-group achieved a 100% medication adherence, as T injections were performed in the doctor’s office and documented. This aspect of treatment is of paramount importance and is considered to be a strength of this study. Thus, long-term TTh in men with hypogonadism appears to be an effective approach to achieve sustained improvements in anthropometric parameters, cardiometabolic function, and risk of CVD events. The low number of CV events observed in the T-group compared with the untreated (control) group strongly suggest that TTh is protective. We believe that the protective effect of T on the CV system provides clinicians with the opportunity to utilize this approach for secondary prevention for men with hypogonadism with a history of CV events.
Written By: Abdulmaged M. Traish, PhD, MBA, Departments of Biochemistry and Urology, Boston University School of Medicine, Boston, MA, USA; Ahmad Haider, MD, Private Urology Practice, Bremerhaven, Germany; Karim Sultan Haider, MD, Private Urology Practice, Bremerhaven, Germany; Gheorghe Doros, PhD, Department of Epidemiology and Statistics, Boston University School of Public Health, Boston, MA, USA; Farid Saad, DVM, PhD, Global Medical Affairs Andrology, Bayer AG, Berlin, Germany & Gulf Medical University, Ajman, United Arab Emirates
Author Contributions:
Dr Traish participated in discussions of study design and data analysis and manuscript writing. Dr Haider participated in study design and involved in conducting the study. Karim Haider participated in data collection and analysis. Dr Doros involved in data statistical analysis and manuscript writing. Dr Saad spear headed the design of the study and involved in data analysis and manuscript writing.
Declaration of Conflicting Interests:
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Farid Saad is a full-time employee of Bayer. Dr. Ahmad Haider has received partial compensation for data entry. Dr. Gheorghe Doros has received payment for statistical analysis.
Funding:
The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: Data entry and statistical analyses were supported by Bayer Pharma.
Declaration of Conflicting Interests:
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Farid Saad is a full-time employee of Bayer. Dr. Ahmad Haider has received partial compensation for data entry. Dr. Gheorghe Doros has received payment for statistical analysis.
Funding:
The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: Data entry and statistical analyses were supported by Bayer Pharma.
References:
1. Traish AM, Zitzmann M. The complex and multifactorial relationship between testosterone deficiency (TD), obesity and vascular disease. Rev Endocr Metab Disord. 2015;16(3):249-268.
2. Traish AM. Adverse health effects of testosterone deficiency (TD) in men. Steroids. 2014;88:106-116.
3. Traish AM. Testosterone and weight loss: the evidence. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):313-322.
4. Kelly DM, Jones TH. Testosterone and obesity. Obes Rev. 2015; 16(7):581-606.
5. Traish AM. Outcomes of testosterone therapy in men with testosterone deficiency (TD): part II. Steroids. 2014;88:117-126.
6. Kelly DM, Jones TH. Testosterone: a vascular hormone in health and disease. J Endocrinol. 2013;217(3):R47-R71.
7. Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol. 2013;217(3):R25-R45.
8. Kelly DM, Jones TH. Testosterone and cardiovascular risk in men. Front Horm Res. 2014;43:1-20.
9. Rao PM, Kelly DM, Jones TH. Testosterone and insulin resistance in the metabolic syndrome and T2DM in men. Nat Rev Endocrinol. 2013;9(8):479-493.
10. Jones TH. Testosterone deficiency: a risk factor for cardiovascular disease? Trends Endocrinol Metab. 2010;21(8):496-506.
11. Yeap BB, Alfonso H, Chubb SA, et al. In older men an optimal plasma testosterone is associated with reduced all-cause mortality and higher dihydrotestosterone with reduced ischemic heart disease mortality, while estradiol levels do not predict mortality. J Clin Endocrinol Metab. 2014;99(1):E9-E18.
12. Morgentaler A, Miner MM, Caliber M, Guay AT, Khera M, Traish AM. Testosterone therapy and cardiovascular risk: advances and controversies. Mayo Clin Proc. 2015;90(2):224-251.
13. Morgentaler A, Zitzmann M, Traish AM, et al. Fundamental concepts regarding testosterone deficiency and treatment: International Expert Consensus Resolutions. May Clin Proc. 2016; 91(7):881-896.
14. Antonio L, Wu FC, O’Neill TW, et al; EMAS Study Group. Associations between sex steroids and the development of metabolic syndrome: a longitudinal study in European men. J Clin Endocrinol Metab. 2015;100(4):1396-1404.
15. Laaksonen DE, Niskanen L, Punnonen K, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27(5): 1036-1041.
16. Ohlsson C, Barrett-Connor E, Bhasin S, et al. High serum testosterone is associated with reduced risk of cardiovascular events in elderly men. The MrOS (osteoporotic fractures in men) study in Sweden. J Am Coll Cardiol. 2011;58(16):1674-1682.
17. Aub JC. The use of testosterone. N Engl J Med. 1940;222(21): 877-881.
18. AubJC,KetySS.Recentadvancesintestosteronetherapy.NEngl J Med. 1943;228(11):338-343.
19. Beaser SB, Massell TB. Therapeutic evaluation of testosterone in peripheral vascular disease. N Engl J Med. 1942;227(2):43-44.
20. Edwards EA, Hamilton JB, Duntley SQ. Testosterone propionate as therapeutic agent in patients with organic disease of the peripheral vessels: preliminary report. N Engl J Med. 1939;220(21): 865.
21. Uricchio JF, Calenda DG. Treatment of angina pectoris. N Engl J Med. 1953;249(17):689-698.
22. Lesser MA. The treatment of angina pectoris with testosterone propionated further observations. N Engl J Med. 1943;228(6): 185-188.
23. Goodman N, Guay A, Dandona P, Dhindsa S, Faiman C, Cunningham GR; AACE Reproductive Endocrinology Scientific Committee. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of testosterone and cardiovascular risk. Endocr Pract. 2015;21(9):1066-1073.
24. Baillargeon J, Urban RJ, Kuo YF, et al. Risk of myocardial Infarction in older men receiving testosterone therapy. Ann Pharmacother. 2014;48(9):1138-1144.
25. Baillargeon J, Urban RJ, Morgentaler A, et al. Risk of venous thromboembolism in men receiving testosterone therapy. Mayo Clin Proc. 2015;90(8):1038-1045.
26. Sharma R, Oni OA, Gupta K, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36(40):2706-2715.
27. Tan RS, Cook KR, Reilly WG. Myocardial infarction and stroke risk in young healthy men treated with injectable testosterone. Int J Endocrinol. 2015;2015:970750.
28. Etminan M, Skeldon SC, Goldenberg SL, Carleton B, Brophy JM. Testosterone therapy and risk of myocardial infarction: a pharmacoepidemiologic study. Pharmacotherapy. 2015;35(1):72-78.
29. Anderson JL, May HT, Lappe ́ DL, et al. Impact of testosterone replacement therapy on myocardial infarction, stroke, and death in men with low testosterone concentrations in an integrated healthcare system. Am J Cardiol. 2016;117(5):794-799.
30. Snyder PJ, Bhasin S, Cunningham GR, et al; Testosterone Trials Investigators. Effects of Testosterone Treatment in Older Men. N Engl J Med. 2016;374(7):611-624.
31. Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010; 363(2):109-122.
32. Vigen R, O’Donnell CI, Baro ́n AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17): 1829-1836. Erratum in: JAMA. 2014;311(9):967.
33. Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013;11:108.
34. Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9(1):e85805.
35. US Food and Drug Administration. Citizen petition denial response from FDA CDER to Public Citizen. 2014. Regulations.gov website. http://www.regulations.gov/#! documentDe tail;D.FDA-2014-P-0258-0003. Accessed August 31, 2014. http://www.citizen.org/documents/2184_FDA%20Denial%20of %20Petition_July%2016,%202014.pdf. Accessed October 5, 2016.
36. Saad F, Haider A, Doros G, Traish A. Long-term treatment of hypogonadal men with testosterone produces substantial and sustained weight loss. Obesity (Silver Spring). 2013;21(10): 1975-1981.
37. Saad F, Yassin A, Doros G, Haider A. Effects of long-term treatment with testosterone on weight and waist size in 411 hypogonadal men with obesity classes I-III: observational data from two registry studies. Int J Obes (Lond). 2016;40(1):162-170.
38. Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2006;154(6): 899-906.
39. Jones TH, Arver S, Behre HM, et al; TIMES2 Investigators. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study). Diabetes Care. 2011;34(4):828-837.
40. Dhindsa S, Prabhakar S, Sethi M, Bandyopadhyay A, Chaudhuri A, Dandona P. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab. 2004;89(11):5462-5468.
41. Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care. 2010;33(6):1186-1192.
42. Francomano D, Bruzziches R, Barbaro G, Lenzi A, Aversa A. Effects of testosterone undecanoate replacement and withdrawal on cardio-metabolic, hormonal and body composition outcomes in severely obese hypogonadal men: a pilot study. J Endocrinol Invest. 2014;37(4):401-411.
43. Hackett G, Cole N, Bhartia M, et al. Blast Study Group. The response to testosterone undecanoate in men with type 2 diabetes is dependent on achieving threshold serum levels (the BLAST study). Int J Clin Pract. 2014;68(2):203-215.
44. Traish AM, Haider A, Doros G, Saad F. Long-term testosterone therapy in hypogonadal men ameliorates elements of the metabolic syndrome: an observational, long-term registry study. Int J Clin Pract. 2014;68(3):314-329.
45. Haider A, Saad F, Doros G, Gooren L. Hypogonadal obese men with and without diabetes mellitus type 2 lose weight and show improvement in cardiovascular risk factors when treated with testosterone: an observational study. Obes Res Clin Pract. 2014; 8(4):e339-e349.
46. Hackett G, Cole N, Bhartia M, Kennedy D, Raju J, Wilkinson P; BLAST Study Group. Testosterone replacement therapy improves metabolic parameters in hypogonadal men with type 2 diabetes but not in men with coexisting depression: the BLAST study. J Sex Med. 2014;11(3):840-856.
47. Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37(5):1199-1208.
48. Dubey RK, Oparil S, Imthum B, Jackson EK. Sex hormones and hypertension. Cardiovasc Res. 2002;53(3):688-708.
49. Svartberg J, von Mu ̈hlen D, Schirmer H, Barrett-Connor E, Sundfjord J, Jorde R. Association of endogenous testosterone with blood pressure and left ventricular mass in men. Tromsø Study. Eur J Endocrinol. 2004;150(1):65-71.
50. Smith JC, Bennett S, Evans LM, et al. The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab. 2001;86(9):4261-4267.
51. Dockery F, Bulpitt CJ, Agarwal S, Donaldson M, Rajkumar C. Testosterone suppression in men with prostate cancer leads to an increase in arterial stiffness and hyperinsulinaemia. Clin Sci. 2003;104(2):195-201.
52. Boyanov MA, Boneva Z, Christov VG. Testosterone supplementation in men with type 2 diabetes, visceral obesity and partial androgen deficiency. Aging Male. 2003;6(1):1-7.
53. Li JY, Zhu JC, Dou JT, et al. Effects of androgen supplementation therapy on partial androgen deficiency in the aging male: a preliminary study. Aging Male. 2002;5(1):47-51.
54. Annemans L, Aristides M, Kubin M. Real-Life Data: A Growing Need. The Official News & Technical Journal of The International Society for Pharmacoeconomics and Outcomes Research. https:// www.ispor.org/news/articles/oct07/rld.asp. Accessed June 1, 2015.
55. Cox JL, Pieper K. Harnessing the power of real-life data. Eur Heart J Suppl. 2015;17(suppl D):D9-D14.
56. Ramasamy R, Scovell J, Mederos M, Ren R, Jain L, Lipshultz L. Association Between Testosterone Supplementation Therapy and Thrombotic Events in Elderly Men. Urology. 2015;86(2):283-285.
57. Shores MM, Smith NL, Forsberg CW, Anawalt BD, Matsumoto AM. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab. 2012;97(6):2050-2058.
58. Muraleedharan V, Marsh H, Kapoor D, Channer KS, Jones TH. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol. 2013;169(6):725-733.
59. Corona G, Maseroli E, Rastrelli G, et al. Cardiovascular risk associated with testosterone-boosting medications: a systematic review and meta-analysis. Expert Opin Drug Saf. 2014;13(10): 1327-1351.
60. Bolon ̃a ER, Uraga MV, Haddad RM, et al. Testosterone use in men with sexual dysfunction: a systematic review and metaanalysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82(1):20-28.
61. Wang C, Swerdloff RS, Iranmanesh A, et al; Testosterone Gel Study Group. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8): 2839-2853.
62. Hackett G, Cole N, Bhartia M, Kennedy D, Raju J, Wilkinson P. Testosterone replacement therapy with long-acting testosterone undecanoate improves sexual function and quality of-life parameters vs. placebo in a population of men with type 2 diabetes. J Sex Med. 2013;10(6):1612-1627.
63. Zitzmann M, Mattern A, Hanisch J, Gooren L, Jones H, Maggi M. IPASS: a Study on the tolerability and effectiveness of injectable testosterone undecanoate for the treatment of male hypogonadism in a worldwide sample of 1438 men. J Sex Med. 2013;10(2): 579-588.
64. Tong SF, Ng CJ, Lee BC, et al. Effect of long-acting testosterone undecanoate treatment on quality of life in men with testosterone deficiency syndrome: a double blind randomized controlled trial. Asian J Androl. 2012;14(4):604-611.
65. Pexman-Fieth C, Behre HM, Morales A, Kan-Dobrosky N, Miller MG. A 6-month observational study of energy, sexual desire, and body proportions in hypogonadal men treated with a testosterone 1% gel. Aging Male. 2014;17(1):1-11.
66. Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95(2):639-650.
67. Aversa A, Bruzziches R, Francomano D, et al. Effects of longacting testosterone undecanoate on bone mineral density in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 36 months controlled study. Aging Male. 2012;15(2):96-102.
68. Aversa A, Bruzziches R, Francomano D, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med. 2010;7(10): 3495-3503.
69. Kalinchenko SY, Tishova YA, Mskhalaya GJ, Gooren LJ, Giltay EJ, Saad F. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled Moscow study. Clin Endocrinol (Oxf). 2010;73(5):602-612.
70. Dhindsa S, Ghanim H, Batra M, et al. Insulin resistance and inflammation in hypogonadotropic hypogonadism and their reduction after testosterone replacement in men with Type 2 diabetes. Diabetes Care. 2016;39(1):82-91.
71. Morgunov LIu, Denisova IA, Rozhkova TI, Stakhovskaia LV, Skvortsova VI. Androgenic deficit and its treatment in stroke male patients with diabetes mellitus type II [in Russian]. Zh Nevrol Psikhiatr Im S S Korsakova. 2011;111(8 pt 2):21-24.
72. Finkelstein JS, Lee H, Burnett-Bowie SA, et al. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369(11):1011-1022.
73. Page ST, Amory JK, Bowman FD, et al. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005;90(3):1502-1510.
74. Bhasin S, Travison TG, Storer TW, et al. Effect of testosterone supplementation with and without a dual 5a-reductase inhibitor on fat-free mass in men with suppressed testosterone production: a randomized controlled trial. JAMA. 2012;307(9):931-939.
75. Nedogoda SV, Barykina IN, Salasyuk AS, Smirnova VO, Khripaeva VJ. Effects of Testosterone Replacement Therapy on Cardio-Metabolic, Hormonal and Anthropometric Parameters in Obese Hypogonadal Men with Metabolic Syndrome. Obesity. 2015;1(2):1-7.
76. Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008; 93(1):68-75.
77. Haring R, Vo ̈lzke H, Steveling A, et al. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20-79. Eur Heart J. 2010; 31(12):1494-1501.
78. Khaw KT, Dowsett M, Folkerd E, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007; 116(23):2694-2701.
79. Corona G, Rastrelli G, Monami M, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol. 2011;165(5):687-701.
80. Menke A, Guallar E, Rohrmann S, et al. Sex steroid hormone concentrations and risk of death in US men. Am J Epidemiol. 2010;171(5):583-592.
81. Tivesten A, Vandenput L, Labrie F, et al. Low serum testosterone and estradiol predict mortality in elderly men. J Clin Endocrinol Metab. 2009;94(7):2482-2488.
82. Pietri P, Vlachopoulos C, Ioakeimidis N, et al. 5B.06: Association of plasma testosterone with central haemodynamics in hypertensive men. J Hypertens. 2015;33(suppl 1):e67.
83. Yassin DJ, Doros G, Hammerer PG, Yassin AA. Long-term testosterone treatment in elderly men with hypogonadism and erectile dysfunction reduces obesity parameters and improves metabolic syndrome and health-related quality of life. J Sex Med. 2014;11(6):1567-1576.
84. Haider A, Yassin A, Doros G, Saad F. Effects of long-term testosterone therapy on patients with “diabesity”: results of observational studies of pooled analyses in obese hypogonadal men with type 2 diabetes. Int J Endocrinol. 2014;2014:683515.
85. Heufelder AE, Saad F, Bunck MC, Gooren L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl. 2009;30(6):726-733.
86. Juang PS, Peng S, Allehmazedeh K, Shah A, Coviello AD, Herbst KL. Testosterone with dutasteride, but not anastrazole, improves insulin sensitivity in young obese men: a randomized controlled trial. J Sex Med. 2014;11(2):563-573.
87. Francomano D, Lenzi A, Aversa A. Effects of five-year treatment with testosterone undecanoate on metabolic and hormonal parameters in ageing men with metabolic syndrome. Int J Endocrinol. 2014;2014:527470.
88. Tambo A, Roshan MH, Pace NP. Testosterone and Cardiovascular Disease. Open Cardiovasc Med J. 2016;10:1-10.
89. Azad N, Pitale S, Barnes WE, Friedman N. Testosterone treatment enhances regional brain perfusion in hypogonadal men. J Clin Endocrinol Metab. 2003;88(7):3064-3068.
90. Pope HG Jr, Cohane GH, Kanayama G, Siegel AJ, Hudson JI. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry. 2003;160(1):105-111.
91. Cohen AT, Goto S, Schreiber K, Torp-Pedersen C. Why do we need observational studies of everyday patients in the real-life setting? Eur Heart J Suppl. 2015;17(suppl D):D2-D8.
92. Wallis CJ, Lo K, Lee Y, et al. Survival and cardiovascular events in men treated with testosterone replacement therapy: an intention-to-treat observational cohort study. Lancet Diabetes Endocrinol. 2016;4(6):498-506.
93. Yialamas MA, Dwyer AA, Hanley E, Lee H, Pitteloud N, Hayes FJ. Acute sex steroid withdrawal reduces insulin sensitivity in healthy men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2007;92(11):4254-4259.
94. Pitteloud N, Mootha VK, Dwyer AA, et al. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care. 2005;28(7):1636-1642.
95. Bleda S, de Haro J, Varela C, Esparza L, Rodriguez J, Acin F. Improving Total-Cholesterol/HDL-Cholesterol Ratio Results in an Endothelial Dysfunction Recovery in Peripheral Artery Disease Patients. Cholesterol. 2012;2012:895326.
96. Rouver WN, Delgado NT, Menezes JB, Santos RL, Moyses MR. Testosterone Replacement Therapy Prevents Alterations of Coronary Vascular Reactivity Caused by Hormone Deficiency Induced by Castration. PLoS One. 2015;10(8):e0137111.
97. Vlachopoulos C, Ioakeimidis N, Miner M, et al. Testosterone deficiency: a determinant of aortic stiffness in men. Atherosclerosis. 2014;233(1):278-283.
98. Lee WC, Kim MT, Ko KT, et al. Relationship between Serum Testosterone and Cardiovascular Disease Risk Determined Using the Framingham Risk Score in Male Patients with Sexual Dysfunction. World J Mens Health. 2014;32(3):139-144.
99. Hoyos CM, Yee BJ, Phillips CL, Machan EA, Grunstein RR, Liu PY. Body compositional and cardiometabolic effects of testosterone therapy in obese men with severe obstructive sleep apnoea: a randomised placebo-controlled trial. Eur J Endocrinol. 2012;167(4):531-541.
100. Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2011;96(9): 2898-2903.
101. Chuang SY, Hsu YY, Chen RC, Liu WL, Pan WH. Abdominal obesity and low skeletal muscle mass jointly predict total mortality and cardiovascular mortality in an elderly Asian population. J Gerontol A BiolSci Med Sci. 2016;71(8): 1049-1055.
102. Huo S, Scialli AR, McGarvey S, et al. Treatment of Men for “Low Testosterone”: A Systematic Review. PLoS One. 2016; 11(9):e0162480. doi:10.1371/journal.pone.0162480.
103. Roovers JP. Registries: what level of evidence do they provide? Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(10):1119-1120.
104. Ng Tang Fui M, Prendergast LA, Dupuis P, et al. Effects of testosterone treatment on body fat and lean mass in obese men on a hypocaloric diet: a randomized controlled trial. BMC Med. 2016;14(1):153.
Go “Beyond the Abstract” - Read an article commentary written by the author
2. Traish AM. Adverse health effects of testosterone deficiency (TD) in men. Steroids. 2014;88:106-116.
3. Traish AM. Testosterone and weight loss: the evidence. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):313-322.
4. Kelly DM, Jones TH. Testosterone and obesity. Obes Rev. 2015; 16(7):581-606.
5. Traish AM. Outcomes of testosterone therapy in men with testosterone deficiency (TD): part II. Steroids. 2014;88:117-126.
6. Kelly DM, Jones TH. Testosterone: a vascular hormone in health and disease. J Endocrinol. 2013;217(3):R47-R71.
7. Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol. 2013;217(3):R25-R45.
8. Kelly DM, Jones TH. Testosterone and cardiovascular risk in men. Front Horm Res. 2014;43:1-20.
9. Rao PM, Kelly DM, Jones TH. Testosterone and insulin resistance in the metabolic syndrome and T2DM in men. Nat Rev Endocrinol. 2013;9(8):479-493.
10. Jones TH. Testosterone deficiency: a risk factor for cardiovascular disease? Trends Endocrinol Metab. 2010;21(8):496-506.
11. Yeap BB, Alfonso H, Chubb SA, et al. In older men an optimal plasma testosterone is associated with reduced all-cause mortality and higher dihydrotestosterone with reduced ischemic heart disease mortality, while estradiol levels do not predict mortality. J Clin Endocrinol Metab. 2014;99(1):E9-E18.
12. Morgentaler A, Miner MM, Caliber M, Guay AT, Khera M, Traish AM. Testosterone therapy and cardiovascular risk: advances and controversies. Mayo Clin Proc. 2015;90(2):224-251.
13. Morgentaler A, Zitzmann M, Traish AM, et al. Fundamental concepts regarding testosterone deficiency and treatment: International Expert Consensus Resolutions. May Clin Proc. 2016; 91(7):881-896.
14. Antonio L, Wu FC, O’Neill TW, et al; EMAS Study Group. Associations between sex steroids and the development of metabolic syndrome: a longitudinal study in European men. J Clin Endocrinol Metab. 2015;100(4):1396-1404.
15. Laaksonen DE, Niskanen L, Punnonen K, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27(5): 1036-1041.
16. Ohlsson C, Barrett-Connor E, Bhasin S, et al. High serum testosterone is associated with reduced risk of cardiovascular events in elderly men. The MrOS (osteoporotic fractures in men) study in Sweden. J Am Coll Cardiol. 2011;58(16):1674-1682.
17. Aub JC. The use of testosterone. N Engl J Med. 1940;222(21): 877-881.
18. AubJC,KetySS.Recentadvancesintestosteronetherapy.NEngl J Med. 1943;228(11):338-343.
19. Beaser SB, Massell TB. Therapeutic evaluation of testosterone in peripheral vascular disease. N Engl J Med. 1942;227(2):43-44.
20. Edwards EA, Hamilton JB, Duntley SQ. Testosterone propionate as therapeutic agent in patients with organic disease of the peripheral vessels: preliminary report. N Engl J Med. 1939;220(21): 865.
21. Uricchio JF, Calenda DG. Treatment of angina pectoris. N Engl J Med. 1953;249(17):689-698.
22. Lesser MA. The treatment of angina pectoris with testosterone propionated further observations. N Engl J Med. 1943;228(6): 185-188.
23. Goodman N, Guay A, Dandona P, Dhindsa S, Faiman C, Cunningham GR; AACE Reproductive Endocrinology Scientific Committee. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of testosterone and cardiovascular risk. Endocr Pract. 2015;21(9):1066-1073.
24. Baillargeon J, Urban RJ, Kuo YF, et al. Risk of myocardial Infarction in older men receiving testosterone therapy. Ann Pharmacother. 2014;48(9):1138-1144.
25. Baillargeon J, Urban RJ, Morgentaler A, et al. Risk of venous thromboembolism in men receiving testosterone therapy. Mayo Clin Proc. 2015;90(8):1038-1045.
26. Sharma R, Oni OA, Gupta K, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36(40):2706-2715.
27. Tan RS, Cook KR, Reilly WG. Myocardial infarction and stroke risk in young healthy men treated with injectable testosterone. Int J Endocrinol. 2015;2015:970750.
28. Etminan M, Skeldon SC, Goldenberg SL, Carleton B, Brophy JM. Testosterone therapy and risk of myocardial infarction: a pharmacoepidemiologic study. Pharmacotherapy. 2015;35(1):72-78.
29. Anderson JL, May HT, Lappe ́ DL, et al. Impact of testosterone replacement therapy on myocardial infarction, stroke, and death in men with low testosterone concentrations in an integrated healthcare system. Am J Cardiol. 2016;117(5):794-799.
30. Snyder PJ, Bhasin S, Cunningham GR, et al; Testosterone Trials Investigators. Effects of Testosterone Treatment in Older Men. N Engl J Med. 2016;374(7):611-624.
31. Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010; 363(2):109-122.
32. Vigen R, O’Donnell CI, Baro ́n AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17): 1829-1836. Erratum in: JAMA. 2014;311(9):967.
33. Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013;11:108.
34. Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9(1):e85805.
35. US Food and Drug Administration. Citizen petition denial response from FDA CDER to Public Citizen. 2014. Regulations.gov website. http://www.regulations.gov/#! documentDe tail;D.FDA-2014-P-0258-0003. Accessed August 31, 2014. http://www.citizen.org/documents/2184_FDA%20Denial%20of %20Petition_July%2016,%202014.pdf. Accessed October 5, 2016.
36. Saad F, Haider A, Doros G, Traish A. Long-term treatment of hypogonadal men with testosterone produces substantial and sustained weight loss. Obesity (Silver Spring). 2013;21(10): 1975-1981.
37. Saad F, Yassin A, Doros G, Haider A. Effects of long-term treatment with testosterone on weight and waist size in 411 hypogonadal men with obesity classes I-III: observational data from two registry studies. Int J Obes (Lond). 2016;40(1):162-170.
38. Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2006;154(6): 899-906.
39. Jones TH, Arver S, Behre HM, et al; TIMES2 Investigators. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study). Diabetes Care. 2011;34(4):828-837.
40. Dhindsa S, Prabhakar S, Sethi M, Bandyopadhyay A, Chaudhuri A, Dandona P. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab. 2004;89(11):5462-5468.
41. Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care. 2010;33(6):1186-1192.
42. Francomano D, Bruzziches R, Barbaro G, Lenzi A, Aversa A. Effects of testosterone undecanoate replacement and withdrawal on cardio-metabolic, hormonal and body composition outcomes in severely obese hypogonadal men: a pilot study. J Endocrinol Invest. 2014;37(4):401-411.
43. Hackett G, Cole N, Bhartia M, et al. Blast Study Group. The response to testosterone undecanoate in men with type 2 diabetes is dependent on achieving threshold serum levels (the BLAST study). Int J Clin Pract. 2014;68(2):203-215.
44. Traish AM, Haider A, Doros G, Saad F. Long-term testosterone therapy in hypogonadal men ameliorates elements of the metabolic syndrome: an observational, long-term registry study. Int J Clin Pract. 2014;68(3):314-329.
45. Haider A, Saad F, Doros G, Gooren L. Hypogonadal obese men with and without diabetes mellitus type 2 lose weight and show improvement in cardiovascular risk factors when treated with testosterone: an observational study. Obes Res Clin Pract. 2014; 8(4):e339-e349.
46. Hackett G, Cole N, Bhartia M, Kennedy D, Raju J, Wilkinson P; BLAST Study Group. Testosterone replacement therapy improves metabolic parameters in hypogonadal men with type 2 diabetes but not in men with coexisting depression: the BLAST study. J Sex Med. 2014;11(3):840-856.
47. Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37(5):1199-1208.
48. Dubey RK, Oparil S, Imthum B, Jackson EK. Sex hormones and hypertension. Cardiovasc Res. 2002;53(3):688-708.
49. Svartberg J, von Mu ̈hlen D, Schirmer H, Barrett-Connor E, Sundfjord J, Jorde R. Association of endogenous testosterone with blood pressure and left ventricular mass in men. Tromsø Study. Eur J Endocrinol. 2004;150(1):65-71.
50. Smith JC, Bennett S, Evans LM, et al. The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab. 2001;86(9):4261-4267.
51. Dockery F, Bulpitt CJ, Agarwal S, Donaldson M, Rajkumar C. Testosterone suppression in men with prostate cancer leads to an increase in arterial stiffness and hyperinsulinaemia. Clin Sci. 2003;104(2):195-201.
52. Boyanov MA, Boneva Z, Christov VG. Testosterone supplementation in men with type 2 diabetes, visceral obesity and partial androgen deficiency. Aging Male. 2003;6(1):1-7.
53. Li JY, Zhu JC, Dou JT, et al. Effects of androgen supplementation therapy on partial androgen deficiency in the aging male: a preliminary study. Aging Male. 2002;5(1):47-51.
54. Annemans L, Aristides M, Kubin M. Real-Life Data: A Growing Need. The Official News & Technical Journal of The International Society for Pharmacoeconomics and Outcomes Research. https:// www.ispor.org/news/articles/oct07/rld.asp. Accessed June 1, 2015.
55. Cox JL, Pieper K. Harnessing the power of real-life data. Eur Heart J Suppl. 2015;17(suppl D):D9-D14.
56. Ramasamy R, Scovell J, Mederos M, Ren R, Jain L, Lipshultz L. Association Between Testosterone Supplementation Therapy and Thrombotic Events in Elderly Men. Urology. 2015;86(2):283-285.
57. Shores MM, Smith NL, Forsberg CW, Anawalt BD, Matsumoto AM. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab. 2012;97(6):2050-2058.
58. Muraleedharan V, Marsh H, Kapoor D, Channer KS, Jones TH. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol. 2013;169(6):725-733.
59. Corona G, Maseroli E, Rastrelli G, et al. Cardiovascular risk associated with testosterone-boosting medications: a systematic review and meta-analysis. Expert Opin Drug Saf. 2014;13(10): 1327-1351.
60. Bolon ̃a ER, Uraga MV, Haddad RM, et al. Testosterone use in men with sexual dysfunction: a systematic review and metaanalysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82(1):20-28.
61. Wang C, Swerdloff RS, Iranmanesh A, et al; Testosterone Gel Study Group. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8): 2839-2853.
62. Hackett G, Cole N, Bhartia M, Kennedy D, Raju J, Wilkinson P. Testosterone replacement therapy with long-acting testosterone undecanoate improves sexual function and quality of-life parameters vs. placebo in a population of men with type 2 diabetes. J Sex Med. 2013;10(6):1612-1627.
63. Zitzmann M, Mattern A, Hanisch J, Gooren L, Jones H, Maggi M. IPASS: a Study on the tolerability and effectiveness of injectable testosterone undecanoate for the treatment of male hypogonadism in a worldwide sample of 1438 men. J Sex Med. 2013;10(2): 579-588.
64. Tong SF, Ng CJ, Lee BC, et al. Effect of long-acting testosterone undecanoate treatment on quality of life in men with testosterone deficiency syndrome: a double blind randomized controlled trial. Asian J Androl. 2012;14(4):604-611.
65. Pexman-Fieth C, Behre HM, Morales A, Kan-Dobrosky N, Miller MG. A 6-month observational study of energy, sexual desire, and body proportions in hypogonadal men treated with a testosterone 1% gel. Aging Male. 2014;17(1):1-11.
66. Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95(2):639-650.
67. Aversa A, Bruzziches R, Francomano D, et al. Effects of longacting testosterone undecanoate on bone mineral density in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 36 months controlled study. Aging Male. 2012;15(2):96-102.
68. Aversa A, Bruzziches R, Francomano D, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med. 2010;7(10): 3495-3503.
69. Kalinchenko SY, Tishova YA, Mskhalaya GJ, Gooren LJ, Giltay EJ, Saad F. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled Moscow study. Clin Endocrinol (Oxf). 2010;73(5):602-612.
70. Dhindsa S, Ghanim H, Batra M, et al. Insulin resistance and inflammation in hypogonadotropic hypogonadism and their reduction after testosterone replacement in men with Type 2 diabetes. Diabetes Care. 2016;39(1):82-91.
71. Morgunov LIu, Denisova IA, Rozhkova TI, Stakhovskaia LV, Skvortsova VI. Androgenic deficit and its treatment in stroke male patients with diabetes mellitus type II [in Russian]. Zh Nevrol Psikhiatr Im S S Korsakova. 2011;111(8 pt 2):21-24.
72. Finkelstein JS, Lee H, Burnett-Bowie SA, et al. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369(11):1011-1022.
73. Page ST, Amory JK, Bowman FD, et al. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005;90(3):1502-1510.
74. Bhasin S, Travison TG, Storer TW, et al. Effect of testosterone supplementation with and without a dual 5a-reductase inhibitor on fat-free mass in men with suppressed testosterone production: a randomized controlled trial. JAMA. 2012;307(9):931-939.
75. Nedogoda SV, Barykina IN, Salasyuk AS, Smirnova VO, Khripaeva VJ. Effects of Testosterone Replacement Therapy on Cardio-Metabolic, Hormonal and Anthropometric Parameters in Obese Hypogonadal Men with Metabolic Syndrome. Obesity. 2015;1(2):1-7.
76. Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008; 93(1):68-75.
77. Haring R, Vo ̈lzke H, Steveling A, et al. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20-79. Eur Heart J. 2010; 31(12):1494-1501.
78. Khaw KT, Dowsett M, Folkerd E, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007; 116(23):2694-2701.
79. Corona G, Rastrelli G, Monami M, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol. 2011;165(5):687-701.
80. Menke A, Guallar E, Rohrmann S, et al. Sex steroid hormone concentrations and risk of death in US men. Am J Epidemiol. 2010;171(5):583-592.
81. Tivesten A, Vandenput L, Labrie F, et al. Low serum testosterone and estradiol predict mortality in elderly men. J Clin Endocrinol Metab. 2009;94(7):2482-2488.
82. Pietri P, Vlachopoulos C, Ioakeimidis N, et al. 5B.06: Association of plasma testosterone with central haemodynamics in hypertensive men. J Hypertens. 2015;33(suppl 1):e67.
83. Yassin DJ, Doros G, Hammerer PG, Yassin AA. Long-term testosterone treatment in elderly men with hypogonadism and erectile dysfunction reduces obesity parameters and improves metabolic syndrome and health-related quality of life. J Sex Med. 2014;11(6):1567-1576.
84. Haider A, Yassin A, Doros G, Saad F. Effects of long-term testosterone therapy on patients with “diabesity”: results of observational studies of pooled analyses in obese hypogonadal men with type 2 diabetes. Int J Endocrinol. 2014;2014:683515.
85. Heufelder AE, Saad F, Bunck MC, Gooren L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl. 2009;30(6):726-733.
86. Juang PS, Peng S, Allehmazedeh K, Shah A, Coviello AD, Herbst KL. Testosterone with dutasteride, but not anastrazole, improves insulin sensitivity in young obese men: a randomized controlled trial. J Sex Med. 2014;11(2):563-573.
87. Francomano D, Lenzi A, Aversa A. Effects of five-year treatment with testosterone undecanoate on metabolic and hormonal parameters in ageing men with metabolic syndrome. Int J Endocrinol. 2014;2014:527470.
88. Tambo A, Roshan MH, Pace NP. Testosterone and Cardiovascular Disease. Open Cardiovasc Med J. 2016;10:1-10.
89. Azad N, Pitale S, Barnes WE, Friedman N. Testosterone treatment enhances regional brain perfusion in hypogonadal men. J Clin Endocrinol Metab. 2003;88(7):3064-3068.
90. Pope HG Jr, Cohane GH, Kanayama G, Siegel AJ, Hudson JI. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry. 2003;160(1):105-111.
91. Cohen AT, Goto S, Schreiber K, Torp-Pedersen C. Why do we need observational studies of everyday patients in the real-life setting? Eur Heart J Suppl. 2015;17(suppl D):D2-D8.
92. Wallis CJ, Lo K, Lee Y, et al. Survival and cardiovascular events in men treated with testosterone replacement therapy: an intention-to-treat observational cohort study. Lancet Diabetes Endocrinol. 2016;4(6):498-506.
93. Yialamas MA, Dwyer AA, Hanley E, Lee H, Pitteloud N, Hayes FJ. Acute sex steroid withdrawal reduces insulin sensitivity in healthy men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2007;92(11):4254-4259.
94. Pitteloud N, Mootha VK, Dwyer AA, et al. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care. 2005;28(7):1636-1642.
95. Bleda S, de Haro J, Varela C, Esparza L, Rodriguez J, Acin F. Improving Total-Cholesterol/HDL-Cholesterol Ratio Results in an Endothelial Dysfunction Recovery in Peripheral Artery Disease Patients. Cholesterol. 2012;2012:895326.
96. Rouver WN, Delgado NT, Menezes JB, Santos RL, Moyses MR. Testosterone Replacement Therapy Prevents Alterations of Coronary Vascular Reactivity Caused by Hormone Deficiency Induced by Castration. PLoS One. 2015;10(8):e0137111.
97. Vlachopoulos C, Ioakeimidis N, Miner M, et al. Testosterone deficiency: a determinant of aortic stiffness in men. Atherosclerosis. 2014;233(1):278-283.
98. Lee WC, Kim MT, Ko KT, et al. Relationship between Serum Testosterone and Cardiovascular Disease Risk Determined Using the Framingham Risk Score in Male Patients with Sexual Dysfunction. World J Mens Health. 2014;32(3):139-144.
99. Hoyos CM, Yee BJ, Phillips CL, Machan EA, Grunstein RR, Liu PY. Body compositional and cardiometabolic effects of testosterone therapy in obese men with severe obstructive sleep apnoea: a randomised placebo-controlled trial. Eur J Endocrinol. 2012;167(4):531-541.
100. Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2011;96(9): 2898-2903.
101. Chuang SY, Hsu YY, Chen RC, Liu WL, Pan WH. Abdominal obesity and low skeletal muscle mass jointly predict total mortality and cardiovascular mortality in an elderly Asian population. J Gerontol A BiolSci Med Sci. 2016;71(8): 1049-1055.
102. Huo S, Scialli AR, McGarvey S, et al. Treatment of Men for “Low Testosterone”: A Systematic Review. PLoS One. 2016; 11(9):e0162480. doi:10.1371/journal.pone.0162480.
103. Roovers JP. Registries: what level of evidence do they provide? Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(10):1119-1120.
104. Ng Tang Fui M, Prendergast LA, Dupuis P, et al. Effects of testosterone treatment on body fat and lean mass in obese men on a hypocaloric diet: a randomized controlled trial. BMC Med. 2016;14(1):153.
Go “Beyond the Abstract” - Read an article commentary written by the author


