Background: Benign prostatic hyperplasia (BPH) and prostate cancer (PCa) are the two predominant urological diseases affecting aging men. Histological findings of BPH have been reported in more than half of men older than 50 years,1 and PCa is both the most common non-skin cancer disease as well as the second most common cause of cancer-related mortality, after lung cancer, in the United States.2 More than 80% of patients with PCa also show histological findings of BPH.3,4 Despite this high prevalence of BPH and PCa, the interaction between these two often coexisting diseases is not well understood. Recent systematic reviews and meta-analyses on both transrectal ultrasound (TRUS) and magnetic resonance imaging (MRI) of the prostate demonstrated a highly significant inverse relationship between BPH/prostate size and the incidence and aggressiveness of PCa.5-7 Furthermore, PCa in patients with large prostates often have a better prognosis when compared to patients with small prostates.8 These clinical observations are the backdrop of an evolving hypothesis suggesting that an increasing prostate/ BPH-size may be protective against PCa development, with specific histo-anatomical changes within the growing prostate as the underlying pathophysiological mechanism.9 In this context it has to be mentioned that the anatomy of the prostate gland is divided into four separate zones, each one with a distinct embryological origin: the central zone (CZ), transition zone (TZ), peripheral zone (PZ), and the anterior fibromuscular stroma (AFS).10 Each zone differs in biological function and clinical significance. It is well documented in the literature that the CZ and TZ are the primary sites for BPH growth, whereas 80-85% of PCa arises in the PZ.11,12 In large BPH prostates, TZ growth causes direct mechanical pressure on the PZ which is trapped and pushed against the prostatic capsule. These histo-morphological changes are commonly found in large prostates. This histo-pathological process causes increased fibrosis of the PZ with secondary atrophy of glandular tissue within the PZ, where the majority of PCa originates. This dynamic interaction between the TZ and PZ in growing BPH prostates may be protective against PCa and could explain the inverse relationship between BPH/prostate size and PCa incidence. This hypothesis has gained more traction with numerous recent studies that have used various methodologies and techniques. This commentary reviews these study results and provides a more comprehensive understanding of the evolving hypothesis on the effect of increasing BPH/prostate size on the development and incidence of PCa. If upcoming studies should confirm the outlined hypothesis of interaction between BPH/prostate size and development of PCa, this would greatly influence future diagnosis and treatment of both disease entities.
Novel measurement of glandular tissue volume via combined MRI and histopathology
Histopathological studies have demonstrated increased capsular fibrosis and glandular atrophy within the PZ of large BPH prostates.13 However, histopathological studies have the limitation of being not precise on defining the boundaries and dimensions of the PZ, where 80-85% of PCa originates. This limitation prevents a precise measurement of glandular tissue density and volume of the PZ. This limitation can be overcome with MRI which is a detailed, systematic imaging technique that enables precise measurement of PZ dimensions, including the critical PZ thickness (PZT) and volume (PZV).14 As outlined in our recent report, by combining MRI with histopathological imaging, it is possible to precisely measure the glandular tissue density (GDPZ) and glandular tissue volume (GVPZ) within the PZ. The glandular tissue volume of the PZ may be a relevant marker for glandular tissue proliferation, and thus PCa development within the PZ. The recent study on combined MRI and histopathological imaging as a novel technique demonstrated a significant decrease in both GDPZ and GVPZ in large prostates.15 As prostate size increased, both GDPZ and GVPZ decreased. These inverse relationships are summarized in Figure 1 (prostate volume vs. GDPZ) and Figure 2 (prostate volume vs. GVPZ). A clear decline in both GDPZ and GVPZ is seen in prostates over 35-40cc, which suggests that patients with smaller prostates have a higher risk of PCa. Additionally, the combined MRI and histopathology approach also showed significantly increased capsule thickness (CapT) associated with increasing prostate volume.15 An increased CapT represents increased fibrosis presumably a consequence of increased pressure by the expanding TZ in large BPH prostates. A previous MRI study in a large cohort also showed a decline of the PZ thickness in large BPH prostates.16 Ultimately, the results of the combined MRI and histopathology technique indicate that this approach, due to the ability of precisely calculating GDPZ and GVPZ, is superior to either MRI or histopathology alone, and may serve as a powerful tool for future research into the relationship between BPH/prostate size and PCa development.
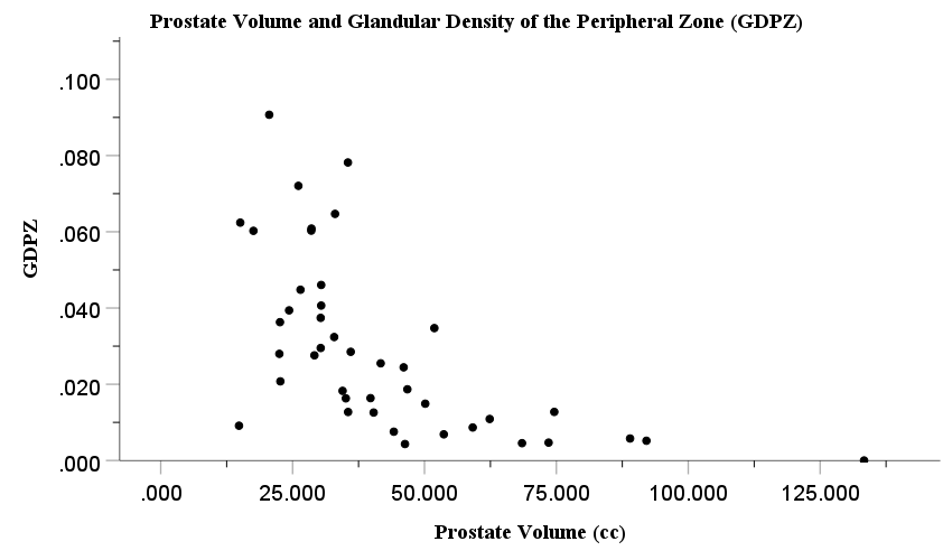
Figure 1 Association between prostate volume (cc) and glandular density of the peripheral zone (GDPZ) (cc).
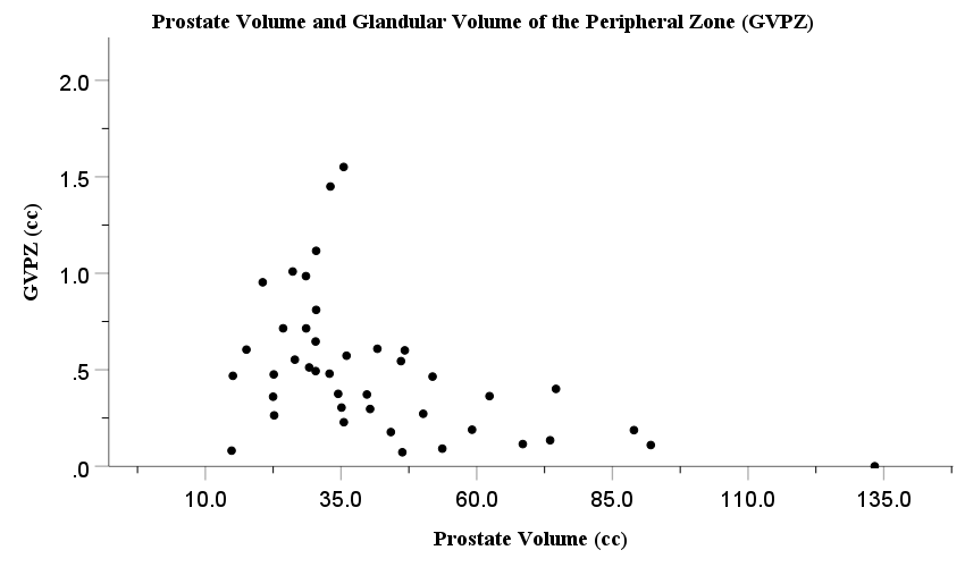
Figure 2 Association between prostate volume (cc) and glandular volume of the peripheral zone (GVPZ) (cc).
Meta-analyses and systemic reviews of transrectal ultrasound and MRI-fusion studies on prostate size and PCa incidence
Three meta-analysis studies on the association between prostate size and PCa incidence reviewed Pubmed-listed articles according to the PRISMA guidelines, and all three reported a significant inverse relationship between prostate volume and PCa incidence. 5,6,7 A review study on TRUS-biopsy cohorts between 1997 and 2021 showed that 95% of the identified articles reported a statistically significant inverse/ negative correlation between prostate volume and incidence of biopsy-proven PCa.7 The remaining studies provided no clear statistically significant conclusions. Not a single study showed a positive correlation between prostate volume and PCa incidence. A systematic review on MRI-Ultrasound fusion biopsy analyzed studies from 2000-2022.6 All of the identified articles showed a significant inverse correlation between prostate volume and PCa incidence, with no studies showing the contrary (a positive correlation between prostate size and PCa incidence). Overall, there is strong emerging evidence that BPH/prostate size and PCa incidence and development are negatively correlated.
Prostate cancer prevention trial (PCPT)
The established inverse correlation between prostate size and PCa incidence, as well as the hypothesis that BPH/prostate size may be protective against PCa development, may explain the findings of the Prostate Cancer Prevention Trial (PCPT). This clinical trial randomly assigned over 18,000 patients to either finasteride, a 5-alpha reductase inhibitor, or placebo.17 Finasteride inhibits the synthesis of dihydrotestosterone (DHT) in the prostate and causes the TZ (where BPH originates) to shrink, this effect is more pronounced in larger prostates. The pre-trial hypothesis was that finasteride may also lower the risk of prostate cancer as DHT is considered the main ‘fuel’ for developing PCa and its level is lowered in the prostate by finasteride. However, the PCPT treatment arm reported a twofold increase in aggressive, high-grade PCa.17 This outcome contradicted the initial idea that Finasteride could serve as ‘chemoprophylaxis’ against PCa by inhibiting dihydrotestosterone; the causes for this phenomenon are still undefined. However, our recent study findings published last month could explain the PCPT study results. The TZ shrinkage under finasteride treatment reduces the mechanical pressure on the PZ. This reduced stress may enable more space for glandular epithelial proliferation in the PZ, which can lead to PCa development.
The above outlined study results support the currently evolving hypothesis that smaller prostates with increased glandular epithelial tissue in the PZ are at higher risk of PCa development, whereas larger BPH prostates with less glandular epithelial tissue volume and increased glandular epithelial tissue atrophy in the PZ are at lower risk.
Conclusion: Multiple studies using different methodologies strongly support the hypothesis that increasing BPH/prostate size provides a protective effect against PCa development. However, there are still some limitations that need to be addressed in future studies:
- This hypothesis assumes that BPH begins earlier in growing/aging prostates than PCa, and not vice versa.
- One limitation of histo-anatomical studies involves the difficulty in reconstructing the anatomy based on histopathology slides as different pathologists use different cutting techniques for acquiring tissue samples. Furthermore, certain slides may need to be excluded due to alteration of zonal architecture by PCa.
- Several studies on histopathology and MRI were based on data from single institutions only. Involvement of multi-institutional samples can increase sample size, statistical power, and generalizability.
Written by: Benjamin Lin, Irina Kim Cavdar, Matthew Buxton, Jake Sellers, Luis Brandi, Naseem Helo, Werner T W de Riese
School of Medicine, Department of Urology, Texas Tech University Health Sciences Center, Lubbock, TX, USA., Department of Radiology, University Medical Center, Lubbock, TX, USA., School of Medicine, Department of Pathology, Texas Tech University Health Sciences Center, Lubbock, TX, USA., School of Medicine, Department of Urology, Texas Tech University Health Sciences Center, Lubbock, TX, USA.
References:
- McVary KT. BPH: epidemiology and comorbidities. Am J Manag Care. Apr 2006;12(5 Suppl):S122-8.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. Jan 2022;72(1):7-33. doi:10.3322/caac.21708
- Liu FC, Hua KC, Lin JR, et al. Prostate resected weight and postoperative prostate cancer incidence after transurethral resection of the prostate: A population-based study. Medicine (Baltimore). Jan 2019;98(3):e13897. doi:10.1097/MD.0000000000013897
- Bostwick DG, Cooner WH, Denis L, et al. The association of benign prostatic hyperplasia and cancer of the prostate. Cancer. Jul 1 1992;70(1 Suppl):291-301. doi:10.1002/1097-0142(19920701)70:1+<291::aid-cncr2820701317>3.0.co;2-4
- Moolupuri A, Camacho J, de Riese WT. Association between prostate size and the incidence of prostate cancer: a meta-analysis and review for urologists and clinicians. Int Urol Nephrol. Oct 2021;53(10):1955-1961. doi:10.1007/s11255-021-02892-w
- Knight AS, Sharma P, de Riese WTW. MRI determined prostate volume and the incidence of prostate cancer on MRI-fusion biopsy: a systemic review of reported data for the last 20 years. Int Urol Nephrol. Aug 30 2022;doi:10.1007/s11255-022-03351-w
- Yamashiro JR, de Riese WTW. Any Correlation Between Prostate Volume and Incidence of Prostate Cancer: A Review of Reported Data for the Last Thirty Years. Res Rep Urol. 2021;13:749-757. doi:10.2147/RRU.S331506
- Al-Khalil S, Ibilibor C, Cammack JT, et al. Association of prostate volume with incidence and aggressiveness of prostate cancer. Res Rep Urol. 2016;8:201-205. doi:10.2147/RRU.S117963
- Sellers J, de Riese WT. Evolving Hypothesis that Prostate/BPH Size Matters in Protection against Prostate Cancer. Exploratory Research and Hypothesis in Medicine. 2022;7(3):179-183. doi:10.14218/ERHM.2022.00028
- McNeal JE. Regional morphology and pathology of the prostate. Am J Clin Pathol. Mar 1968;49(3):347-57. doi:10.1093/ajcp/49.3.347
- Augustin H, Erbersdobler A, Hammerer PG, et al. Prostate cancers in the transition zone: Part 2; clinical aspects. BJU Int. Dec 2004;94(9):1226-9. doi:10.1111/j.1464-410X.2004.05147.x
- Alcaraz A, Hammerer P, Tubaro A, et al. Is there evidence of a relationship between benign prostatic hyperplasia and prostate cancer? Findings of a literature review. Eur Urol. Apr 2009;55(4):864-73. doi:10.1016/j.eururo.2008.11.011
- Sellers J, Ward E, Weaver P, et al. Association of prostate size with capsule thickness and glandular epithelial cell density: The possible clinical implications on prostate cancer development. Journal of Clinical Urology. 0(0):20514158221086399. doi:10.1177/20514158221086399
- Peng Y, Shen D, Liao S, et al. MRI-based prostate volume-adjusted prostate-specific antigen in the diagnosis of prostate cancer. J Magn Reson Imaging. Dec 2015;42(6):1733-9. doi:10.1002/jmri.24944
- Lin B, Cavdar IK, Buxton M, et al. Association between prostate size and glandular tissue volume of the peripheral zone via novel combined MRI and histopathology: possible pathophysiological implications on prostate cancer development. Int Urol Nephrol. Feb 4 2023;doi:10.1007/s11255-023-03483-7
- Sellers J, Wagstaff R, Helo N, et al. Association Between Prostate Size and MRI Determined Quantitative Prostate Zonal Measurements. Res Rep Urol. 2022;14:265-274. doi:10.2147/rru.S362070
- Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. Jul 17 2003;349(3):215-24. doi:10.1056/NEJMoa030660


