Traditional models for studying BPH have fallen short in replicating the human disease's complexity, thereby impeding the development of effective treatments.2,3 Our recently published research introduces a novel patient-derived xenograft (PDX) model that retains the morphological, histological, and molecular characteristics of benign prostatic tissues, offering a more accurate platform for understanding BPH and evaluating potential therapies.
We established this model by collecting paired BPH (transition zone) and normal (peripheral zone) tissue cores from eight patients undergoing cystoprostatectomies. The tissue cores were subsequently, weighed, sliced, and implanted under the renal capsules of immunodeficient mice. Over the course of three months, we assessed changes in PDX weight, architecture, cellular proliferation, apoptosis, transcriptomics, and proteomics.
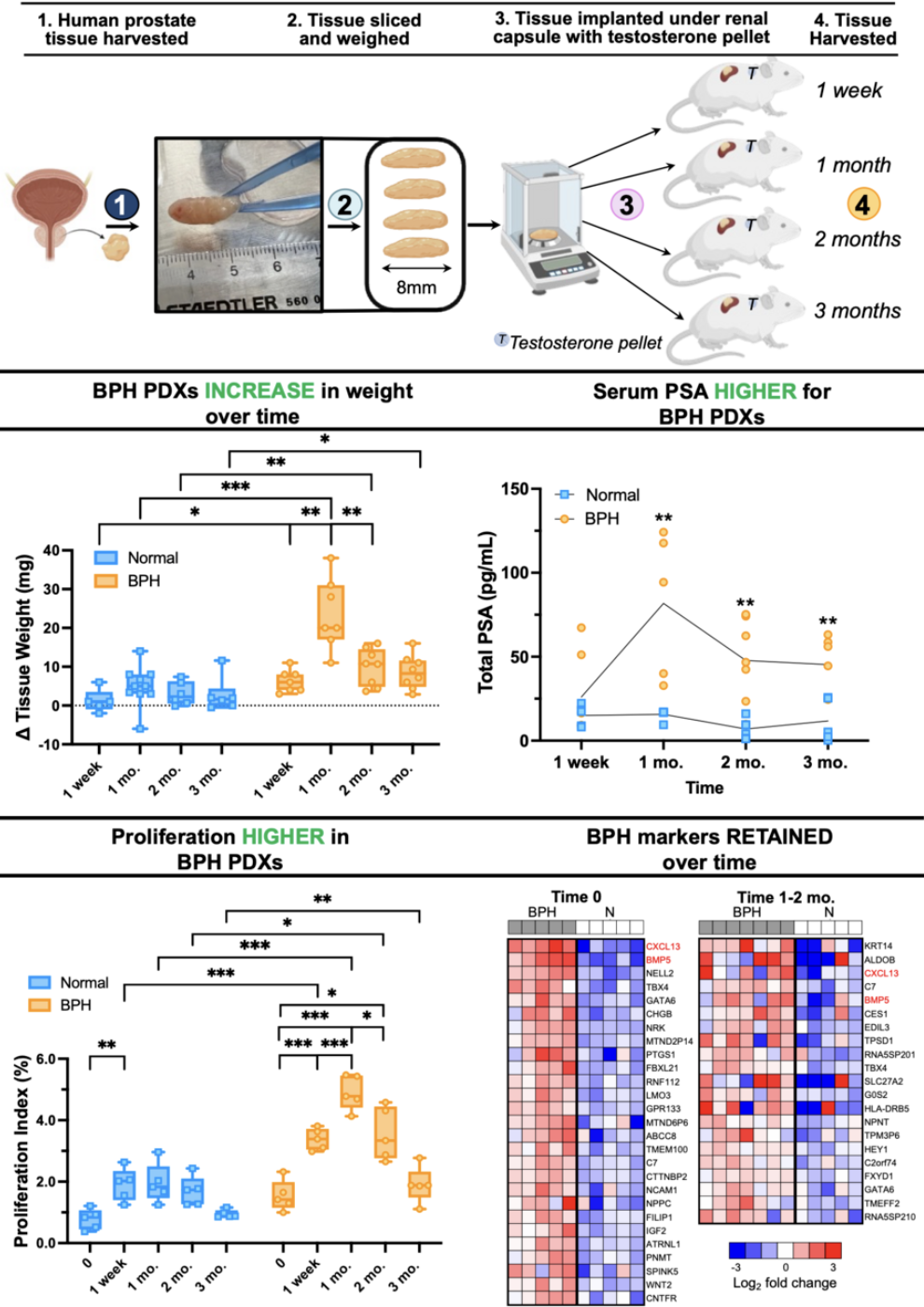
One of the most striking findings from this study was that, unlike normal tissues, BPH tissues grew significantly as well as maintained their structural integrity and proliferative capacity under the renal capsule. In contrast, the normal tissues remained largely unchanged over time. This was particularly evident when we treated BPH PDXs with finasteride, a standard of care therapy for BPH. The treated BPH PDXs showed a significant decrease in proliferation and serum total PSA (from the mice hosting the grafts) and increase in apoptosis, aligning with the therapeutic outcomes observed in clinical settings.
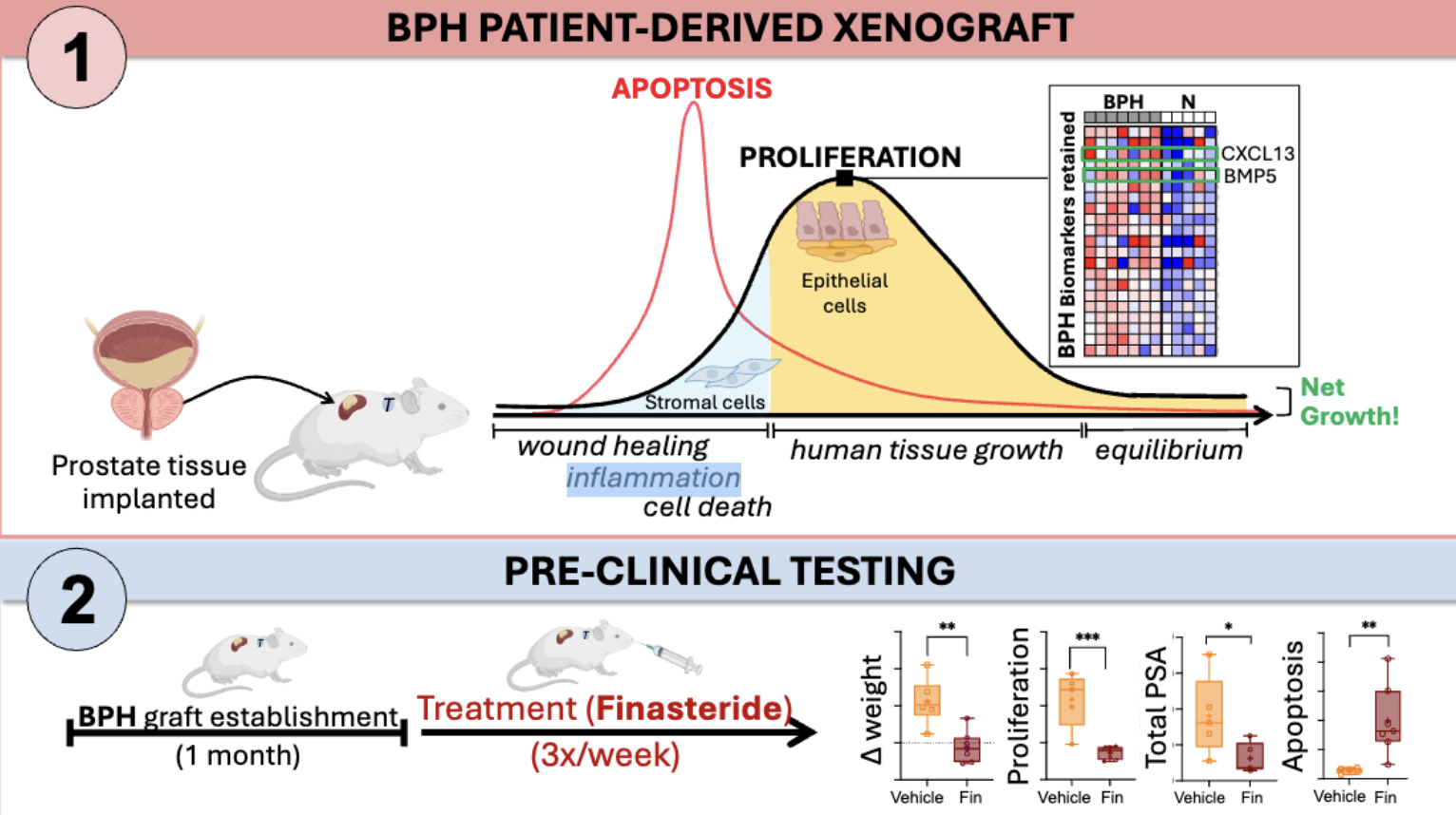
Unique to this model, we also utilized molecular profiling to evaluate the BPH PDXs over time to reveal specific gene and protein expression patterns correlating with BPH pathophysiology. These analyses showed that stromal cells initially proliferate, followed by a subsequent growth of epithelial cells. This is especially interesting as stromal proliferation is posited as a driver of BPH initiation and both embryonic mesenchyme as well as adult reactive stroma myofibroblasts have been shown to wield regulatory control over epithelial proliferation and differentiation. Additionally, we showed the retention of BPH-specific biomarkers and how these markers evolved over time. For example, an increased immune and stress response was observed at 1 week, followed by increased expression of proliferation markers and BPH-specific stromal signaling molecules, such as BMP5 and CXCL13, at 1 month. This was encouraging as BMP5 is a stromal signaling molecule that is overexpressed in individuals with BPH.4,5 Graft stabilization to pre-implant characteristics was apparent between 2 and 3 months. This dynamic response highlights the PDX model's utility in capturing the transient and retained features that occur during BPH progression.
Our research has opened a new avenue for studying BPH by providing a model that mimics the human condition with fidelity. It serves as a tool to evaluate molecularly targeted therapies for pre-clinical testing and identify the underlying mechanism of actions of therapeutic agents for BPH such using multiomic analyses. The insights gained from this study lay a foundation for future investigations into the biological underpinnings of BPH, potentially leading to more targeted and effective treatments.
Written by:
- Alexandra L. Polasko, MD, Department of Urology, Stanford University, Stanford, CA
- James D. Brooks, MD, Department of Urology, Canary Center at Stanford for Cancer Early Detection, Stanford University, Stanford, CA
- Chughtai, B., J. C. Forde, D. D. M. Thomas, L. Laor, T. Hossack, H. H. Woo, A. E. Te and S. A. Kaplan. Benign prostatic hyperplasia. Nat. Rev. Dis. Primers, 2016, 2, 16031.10.1038/nrdp.2016.31
- Devlin, C. M., M. S. Simms and N. J. Maitland. Benign prostatic hyperplasia - what do we know? BJU Int, 2021, 127, 389-399.10.1111/bju.15229
- Zhang, J., M. Zhang, J. Tang, G. Yin, Z. Long, L. He, C. Zhou, L. Luo, L. Qi and L. Wang. Animal models of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis, 2021, 24, 49-57.10.1038/s41391-020-00277-1
- Luo, J., T. Dunn, C. Ewing, J. Sauvageot, Y. Chen, J. Trent and W. Isaacs. Gene expression signature of benign prostatic hyperplasia revealed by cDNA microarray analysis. Prostate, 2002, 51, 189-200.10.1002/pros.10087
- Middleton, L. W., Z. Shen, S. Varma, A. S. Pollack, X. Gong, S. Zhu, C. Zhu, J. W. Foley, S. Vennam and R. T. Sweeney. Genomic analysis of benign prostatic hyperplasia implicates cellular relandscaping in disease pathogenesis. JCI insight, 2019, 4.10.1172/jci.insight.129749


