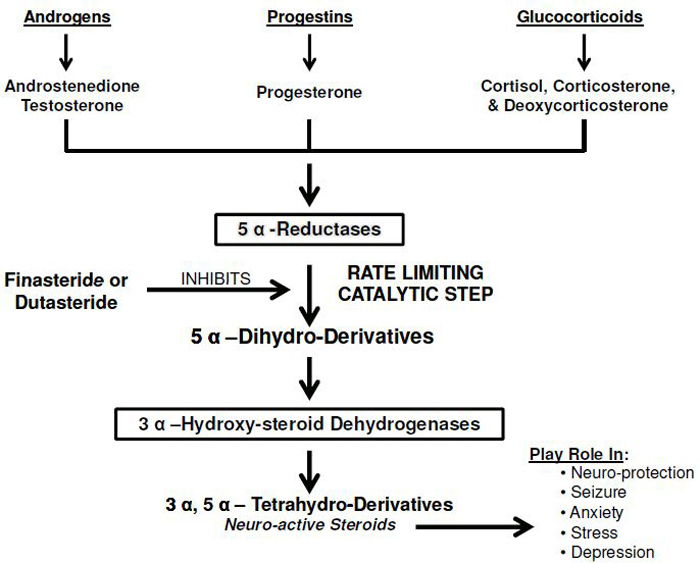
BERKELEY, CA (UroToday.com) - Two drugs, namely finasteride and dutasteride, were developed as specific 5α-reductase inhibitors (5α-RIs) and were approved by the FDA for the treatment of BPH symptoms. These agents have proven useful in reducing urinary retention and minimizing surgical intervention in patients with benign prostatic hyperplasia (BPH) symptoms, and considerable literature exists describing the benefits of these agents. 5α-Rs isoforms (types 1, 2 and 3) are widely distributed in many tissues including the central nervous system, and inhibition of these enzymes results in blockade of synthesis of several key hormones and neuro-active steroids (Figure) leading to a host of adverse effects, including loss of or reduced libido, erectile dysfunction, orgasmic dysfunction, increased high Gleason grade prostate cancer (PCa), observed heart failure and cardiovascular events in clinical trials, and depression. Physicians need to be aware of potential adverse effects and communicate such information to their patients prior to commencing 5α-RIs therapy.
I. Adverse Effects of 5α-Rerducase inhibitors’ Therapy on Sexual Function
In animal studies, administration of 5α-RI, blocked the stimulatory effects of T propionate (TP) on erection in castrated rats.[8, 9] Administration of 5α-DHT with or without the 5α-RIs, restored sexual behavior in long-term castrated male rats suggesting a critical role for 5α-DHT in erectile physiology.[10] Öztekin et al.,[11] and Pinsky et al.,[12] demonstrated that treatment of mature animals with dutasteride resulted in reduced serum DHT levels by ~86.5% after 30 days. The intracavernosal pressure (ICP) decreased significantly in animals treated with dutasteride. More profoundly, connective tissue deposition was markedly increased in the corpus cavernosum of the dutasteride-treated animals. Concomitant with these changes are the markedly reduced expression of neuronal nitric oxide synthase (nNOS) and increased expression of the inducible NOS (iNOS). Zhang et al.,[13] reported that treatment of male mature animals for 16 weeks with a daily oral dose of 4.5 mg/kg finasteride significantly reduced 5α-DHT levels and attenuated penile erectile response to electrical field stimulation of the cavernous nerve. This treatment also reduced the trabecular smooth muscle content and increased connective tissue deposition, reminiscent of the data reported by Traish et al., in the castrated animal model.[14] In addition, finasteride treatment reduced endothelial nitric oxide synthase (eNOS) expression.
In men, ED was consistently noted in observational studies as well as in double-blind, randomized, placebo controlled trials. Roehrborn et al.,[15] and Siami et al.,[16] reported that approximately 6% of the patients reported ED in a two-year follow up to the CombAT trial.[15, 16] Hudson et al.,[17] reported that ED occurred in 6.7% and 4.0% of patients treated with dutasteride or placebo, respectively, in a trial for treatment of BPH.[17] Desgrandchamps et al.,[18] reported 7% of the drug related adverse effects were ED. In the PROSPECT study, ED was established but determined subjectively in an open-ended interview.[19] In a recent study Fwu et al.,[20] investigated the effects of finasteride -- with or without combined therapy with alpha blockers -- on sexual function and found that men assigned to finasteride or finasteride combined with doxazosin experienced a worsening of several domains of sexual function compared to placebo.[20]
II. 5α-Reductase Inhibitors Therapy Contributes to High Gleason Grade PCa
There is no evidence that T or 5α-DHT causes initiation, promotion, or development of PCa.[21, 22, 23, 24] This notion was further supported by recent data from Muller et al.,[24] in which patients enrolled in the placebo arm of the REDUCE trial[3] showed no association between baseline total T or 5α-DHT and PCa. The authors concluded that “baseline serum T and 5α-DHT levels were unrelated to prostate cancer detection or grade.”[3, 24] This raises the question – how could 5α-reductase inhibitors be useful as chemo-preventative agents if PCa development is unrelated to T or 5αDHT? As stated by Walsh in the FDA panel discussion in 2010, “No clinical benefit has been demonstrated in patients with prostate cancer treated with PROSCAR”.[25] In addition, the potential reduction in the incidence of PCa in the general population with the use of these agents may not exceed 10%, which is not statistically significant.[25] Furthermore, the documented increase in Gleason high-grade PCa tumors in response to treatment with these agents[26] proves that these drugs do not prevent development and growth of PCa; they merely prevent biopsies due to reduction in prostate volume. Most importantly, if 5α-RIs indeed prevent PCa development, why are these drugs not approved for treatment of PCa? No improvement in survival rates was noted in patients treated with finasteride vs placebo, suggesting that these drugs provide no advantages in chemoprevention of PCa.[27]
III. Potential Adverse Effects of 5a-Reductase Therapy on the Central Nervous System (CNS)
Neuroactive steroids elicit important neuroprotective effects during trauma and injury to the central nervous system.[28] Inhibition of 5α-R by finasteride is thought to contribute to reduced neuroplasticity due to structural changes resulting from inhibition of neurogenesis in the hippocampus. Finasteride treatment in mice showed decreased cell proliferation in the hippocampus, suggesting that inhibitors of 5α-R block neurogenesis.[29] It has been reported that allopregnanolone (AP) levels were significantly decreased in post-mortem human brains of Alzheimer’s disease (AD) patients[30] An inverse correlation was noted between AP levels and the degree of neurological degeneration in pathological section of AD patients.[30] We speculate that 5α-RIs may contribute to reduced levels of neurosteroids in the CNS and this may enhance the progression of neurodegenerative disease, such as AD.
Epilepsy/Convulsion:
Progesterone (P) is an effective anti-convulsing agent in humans.[31] The anticonvulsive properties of progesterone diminished when animals were treated with finasteride. In a mouse model of pentylenetetrazol-induced seizures, there was an approximate 50% decrease in the protective effect of progesterone in mice when treated with finasteride.[32] Higher dosages of finasteride produced more persistent symptoms of pentylenetetrazol-induced seizures.[32] However, when AP was administered together with finasteride, the pentylenetetrazol seizure activity was reversed. This finding indicates that the anti-seizure properties of progesterone are attributed to its metabolite AP emphasizing the critical role of 5 α-Rs.[32, 33, 34]
Depression:
Anxiety is often found as a co-morbidity of depression. The administration of AP produces antidepressant and anxiolytic effects.[35, 36] Co-administration of finasteride and progesterone blocked progesterone’s anxiolytic effect.[37] This finding suggests that a metabolite of progesterone is responsible for the anxiety reducing effect of progesterone. An inverse relationship between levels of AP and depression has been demonstrated in male patients with depression.[38] Pre-clinical studies have suggested that reduction in AP levels by 5α-RIs may contribute to depressive symptoms.[39] Increased depressive symptoms are thought to be linked to finasteride treatment.[40] A statistically significant correlation was observed between use of finasteride and depressive symptoms.[41] Persistent side effects have been noted even after discontinuation of finasteride treatment[42, 43] from 3 months to 11 years, suggesting that the adverse effects of finasteride may be permanent.[40]
Summary
A substantial body of evidence exists which points to serious health effects of 5α-RIs’ therapy. These include loss or reduced libido, erectile dysfunction, orgasmic and ejaculatory dysfunction,[44, 45] development of high grade prostate cancer tumors, potential negative cardiovascular events, and depression. The argument that the benefits of these drugs outweigh the risks is slowly eroding in the face of new emerging scientific evidence from pre-clinical and clinical studies. The available data demonstrate that such drugs do pose serious adverse effects, especially in a subset of men who may have the predisposition to be affected severely. Physicians need to be aware of the adverse side effects of these drugs and are encouraged to share this information with their patients prior to commencing therapy with finasteride or dutasteride.
References:
- Traish AM. 5alpha-reductases in human physiology: an unfolding story. Endocr Pract. 2012;18: 965-975.
- Traish AM, Hassani J, Guay AT, Zitzmann M, Hansen ML. Adverse side effects of 5alpha-reductase inhibitors therapy: persistent diminished libido and erectile dysfunction and depression in a subset of patients. J Sex Med. 2011;8: 872-884.
- Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, Pettaway CA, Tammela TL, Teloken C, Tindall DJ, Somerville MC, Wilson TH, Fowler IL, Rittmaster RS. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362: 1192-1202.
- Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, Lieber MM, Cespedes RD, Atkins JN, Lippman SM, Carlin SM, Ryan A, Szczepanek CM, Crowley JJ, Coltman CA, Jr. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349: 215-224.
- Wessells H, Roy J, Bannow J, Grayhack J, Matsumoto AM, Tenover L, Herlihy R, Fitch W, Labasky R, Auerbach S, Parra R, Rajfer J, Culbertson J, Lee M, Bach MA, Waldstreicher J. Incidence and severity of sexual adverse experiences in finasteride and placebo-treated men with benign prostatic hyperplasia. Urology. 2003;61: 579-584.
- Irwig MS, Kolukula S. Persistent sexual side effects of finasteride for male pattern hair loss. J Sex Med. 2011;8: 1747-1753.
- Irwig MS. Persistent sexual side effects of finasteride: could they be permanent? J Sex Med. 2012;9: 2927-2932.
- Bradshaw WG, Baum MJ, Awh CC. Attenuation by a 5 alpha-reductase inhibitor of the activational effect of testosterone propionate on penile erections in castrated male rats. Endocrinology. 1981;109: 1047-1051.
- Saksena SK, Lau IF, Chang MC. The inhibition of the conversion of testosterone into 5alpha-dihydrotestosterone in the reproductive organs of the male rat. Steroids. 1976;27: 751-757.
- Baum MJ. A comparison of the effects of methyltrienolone (R 1881) and 5 alpha-dihydrotestosterone on sexual behavior of castrated male rats. Horm Behav. 1979;13: 165-174.
- Oztekin CV, Gur S, Abdulkadir NA, Lokman U, Akdemir AO, Cetinkaya M, Hellstrom WJ. Incomplete recovery of erectile function in rat after discontinuation of dual 5-alpha reductase inhibitor therapy. J Sex Med. 2012;9: 1773-1781.
- Pinsky MR, Gur S, Tracey AJ, Harbin A, Hellstrom WJ. The effects of chronic 5-alpha-reductase inhibitor (dutasteride) treatment on rat erectile function. J Sex Med. 2011;8: 3066-3074.
- Zhang MG, Wang XJ, Shen ZJ, Gao PJ. Long-term oral administration of 5alpha-reductase inhibitor attenuates erectile function by inhibiting autophagy and promoting apoptosis of smooth muscle cells in corpus cavernosum of aged rats. Urology. 2013;82: 743 e9 -15.
- Traish AM, Park K, Dhir V, Kim NN, Moreland RB, Goldstein I. Effects of castration and androgen replacement on erectile function in a rabbit model. Endocrinology. 1999;140:1861-1868.
- Roehrborn CG, Siami P, Barkin J, Damiao R, Major-Walker K, Morrill B, Montorsi F. The effects of dutasteride, tamsulosin and combination therapy on lower urinary tract symptoms in men with benign prostatic hyperplasia and prostatic enlargement: 2-year results from the CombAT study. J Urol. 2008;179: 616-621; discussion 21.
- Siami P, Roehrborn CG, Barkin J, Damiao R, Wyczolkowski M, Duggan A, Major-Walker K, Morrill BB. Combination therapy with dutasteride and tamsulosin in men with moderate-to-severe benign prostatic hyperplasia and prostate enlargement: the CombAT (Combination of Avodart and Tamsulosin) trial rationale and study design. Contemp Clin Trials. 2007;28: 770-779.
- Hudson PB, Boake R, Trachtenberg J, Romas NA, Rosenblatt S, Narayan P, Geller J, Lieber MM, Elhilali M, Norman R, Patterson L, Perreault JP, Malek GH, Bruskewitz RC, Roy JB, Ko A, Jacobsen CA, Stoner E. Efficacy of finasteride is maintained in patients with benign prostatic hyperplasia treated for 5 years. The North American Finasteride Study Group. Urology. 1999;53: 690-695.
- Desgrandchamps F, Droupy S, Irani J, Saussine C, Comenducci A. Effect of dutasteride on the symptoms of benign prostatic hyperplasia, and patient quality of life and discomfort, in clinical practice. BJU Int. 2006;98: 83-88.
- Canguven O, Burnett AL. The effect of 5 alpha-reductase inhibitors on erectile function. J Androl. 2008;29: 514-523.
- Fwu CW, Eggers PW, Kirkali Z2, McVary KT, Burrows PK, Kusek JW. Change in sexual function in men with lower urinary tract symptoms (LUTS)/ benign prostatic hyperplasia (BPH) associated with long-term treatment with doxazosin, finasteride, and combined therapy. J Urol. 2013 Dec 13 (in press)
- Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol. 2009; 55: 310-320.
- Traish AM, Morgentaler A. Epidermal growth factor receptor expression escapes androgen regulation in prostate cancer: a potential molecular switch for tumour growth. Br J Cancer. 2009;101: 1949-1956.
- Morgentaler A. Goodbye androgen hypothesis, hello saturation model. Eur Urol. 2012;62: 765-767.
- Muller RL, Gerber L, Moreira DM, Andriole G, Castro-Santamaria R, Freedland SJ. Serum testosterone and dihydrotestosterone and prostate cancer risk in the placebo arm of the Reduction by Dutasteride of Prostate Cancer Events trial. Eur Urol. 2012;62: 757-764.
- Food and Drug Administration. Oncologic Drugs Advisory Committee - 2010 Meeting Materials, Oncologic Drugs Advisory Committee. 2010.
- Theoret MR, Ning YM, Zhang JJ, Justice R, Keegan P, Pazdur R. The risks and benefits of 5alpha-reductase inhibitors for prostate-cancer prevention. N Engl J Med. 2011;365: 97-99.
- Thompson IM, Jr., Goodman PJ, Tangen CM, Parnes HL, Minasian LM, Godley PA, Lucia MS, Ford LG. Long-term survival of participants in the prostate cancer prevention trial. N Engl J Med. 2013;369: 603-610.
- Melcangi RC, Panzica GC. Allopregnanolone: State of the art. Prog Neurobiol. 2013.
- Romer B, Pfeiffer N, Lewicka S, Ben-Abdallah N, Vogt MA, Deuschle M, Vollmayr B, Gass P. Finasteride treatment inhibits adult hippocampal neurogenesis in male mice. Pharmacopsychiatry. 2010;43: 174-178.
- Naylor JC, Kilts JD, Hulette CM, Steffens DC, Blazer DG, Ervin JF, Strauss JL, Allen TB, Massing MW, Payne VM, Youssef NA, Shampine LJ, Marx CE. Allopregnanolone levels are reduced in temporal cortex in patients with Alzheimer's disease compared to cognitively intact control subjects. Biochim Biophys Acta. 2010;1801: 951-959.
- Singh S, Hota D, Prakash A, Khanduja KL, Arora SK, Chakrabarti A. Allopregnanolone, the active metabolite of progesterone protects against neuronal damage in picrotoxin-induced seizure model in mice. Pharmacol Biochem Behav. 2010;94: 416-422.
- Kokate TG, Banks MK, Magee T, Yamaguchi S, Rogawski MA. Finasteride, a 5alpha-reductase inhibitor, blocks the anticonvulsant activity of progesterone in mice. J Pharmacol Exp Ther. 1999;288: 679-684.
- Lonsdale D, Burnham WM. The anticonvulsant effects of progesterone and 5alpha-dihydroprogesterone on amygdala-kindled seizures in rats. Epilepsia. 2003;44: 1494-1499.
- Reddy DS, Castaneda DC, O'Malley BW, Rogawski MA. Anticonvulsant activity of progesterone and neurosteroids in progesterone receptor knockout mice. J Pharmacol Exp Ther. 2004;310: 230-239.
- Kenny B, Ballard S, Blagg J, Fox D. Pharmacological options in the treatment of benign prostatic hyperplasia. J Med Chem. 1997;40: 1293-1315.
- Khisti RT, Chopde CT, Jain SP. Antidepressant-like effect of the neurosteroid 3alpha-hydroxy-5alpha-pregnan-20-one in mice forced swim test. Pharmacol Biochem Behav. 2000;67: 137-143.
- Bitran D, Shiekh M, McLeod M. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAA receptors. J Neuroendocrinol. 1995;7: 171-177.
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci U S A. 1998;95: 3239-3244.
- Le Melledo JM, Baker G. Role of progesterone and other neuroactive steroids in anxiety disorders. Expert Rev Neurother. 2004;4: 851-860.
- Irwig MS. Depressive symptoms and suicidal thoughts among former users of finasteride with persistent sexual side effects. J Clin Psychiatry. 2012; 73: 1220-1223.
- Rahimi-Ardabili B, Pourandarjani R, Habibollahi P, Mualeki A. Finasteride induced depression: a prospective study. BMC Clin Pharmacol. 2006; 6: 7.
- Melcangi RC, Caruso D, Abbiati F, Giatti S, Calabrese D, Piazza F, Cavaletti G. Neuroactive steroid levels are modified in cerebrospinal fluid and plasma of post-finasteride patients showing persistent sexual side effects and anxious/depressive symptomatology. J Sex Med. 2013;10: 2598-2603.
- Caruso D, Abbiati F, Giatti S, Romano S, Fusco L, Cavaletti G, Melcangi RC. Patients treated for male pattern hair with finasteride show, after discontinuation of the drug, altered levels of neuroactive steroids in cerebrospinal fluid and plasma. J Steroid Biochem Mol Biol. 2014 Apr 6. [Epub ahead of print]
- Gubelin Harcha W, Barboza Martínez J, Tsai TF, Katsuoka K, Kawashima M, Tsuboi R, Barnes A, Ferron-Brady G, Chetty D. A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. J Am Acad Dermatol. 2014: S0190-9622:01171-7.
- Irwig MS. Persistent sexual and non-sexual adverse effects of finasteride in younger men. Sex Med Rev. 2014; 2: 24–35.
Written by:
Abdulmaged M. Traish, PhD as part of Beyond the Abstract on UroToday.com. This initiative offers a method of publishing for the professional urology community. Authors are given an opportunity to expand on the circumstances, limitations etc... of their research by referencing the published abstract.
Professor of Biochemistry
Professor of Urology
Research Director,
Institute of Sexual Medicine
Boston University School of Medicine
Boston, MA USA


