The existing framework for the management of over-active bladder symptoms (OAB) (urge, frequency, nocturia) is essentially what has been defined and recommended by the ICS (International Continence Society)1. OAB symptoms in women are said to be associated with chronic pelvic pain (CPP), bowel dysfunction and organ prolapse, but no numerical relationship has been stated 1. The pathogenesis of OAB is not stated by ICS. However, the 5th International Consultation 2 states that “OAB (symptomatic diagnosis) is often assumed to be caused by detrusor overactivity (DO; urodynamic diagnosis), even if this does not always seem to be the case”. Regarding the treatment of OAB, the Consultation states, “Many drugs have been tried, but the results are often disappointing, partly due to poor treatment efficacy and/or side effects” 2. No surgical treatment for OAB is mentioned in the Incontinence 5th International Consultation Paris Feb 2012.
This work is based on the Integral Theory 3 which considers OAB symptoms to be a premature activation of an otherwise normal micturition reflex 4,5 being a secondary manifestation of looseness in the vagina or its suspensory ligaments, due to altered collagen/elastin 3. In this context, OAB conditions are considered to be potentially curable surgically by using strips of tape to create collagenous neoligaments 6. In previous studies, 7,8 a surgical cure of urge incontinence had been reported using a posterior sling which works by suspending the uterine apex. This posterior sling operation is performed via the perineum. It cannot support the cardinal ligament which is considered a key supporting structure of the uterus and, most importantly, the stretch receptors of the bladder base 3. The problem of cardinal ligament reconstruction was solved by TFS which is uniquely capable of reattaching the uterus to the lateral wall of the pelvis to cure high cystocele, another important cause of urge symptoms 9.
According to the Theory, the cardinal/uterosacral ligaments are key insertion points for the directional vectors which stretch the vaginal membrane to prevent premature activation of the micturition reflex, in which defects are manifested as ‘OAB’ symptoms. Six centres agreed to submit prospective case control data concerning the fate of OAB and other symptoms for 12 months in patients having TFS tensioned mini sling 9,10 repair of the cardinal ligament and TFS uterosacral ligament operations for cure of apical prolapse.
The primary aims of this study were to prospectively track the fate of OAB and other pelvic symptoms, as well as determining the effectiveness of the TFS regarding apical prolapse repair. Secondary aims were to determine proportional links between pelvic floor symptoms and the relative effectiveness of the TFS with respect to the improvement of each symptom.
Material and Methods
This is a prospective case report study comprising of 611 patients from 6 Female Pelvic Floor tertiary referral centres in Germany, Australia and Japan carried out mainly between January 2009 to January 2012, with some data from 2005. During the first consultation, all patients completed the ITSQ (Integral System pelvic symptom questionnaire) 11 validated and self-administered to remove bias. The ITSQ is the basis for the Pictorial Algo-rithm (Figure 2), which guided diagnosis and sur-gery of ligament damage. Patients were examined to assess the degree of prolapse and confirm spe-cific ligament defects. Other symptoms known to be associated with OAB, such as chronic pelvic pain and fecal incontinence 1, were recorded and tracked prospectively.
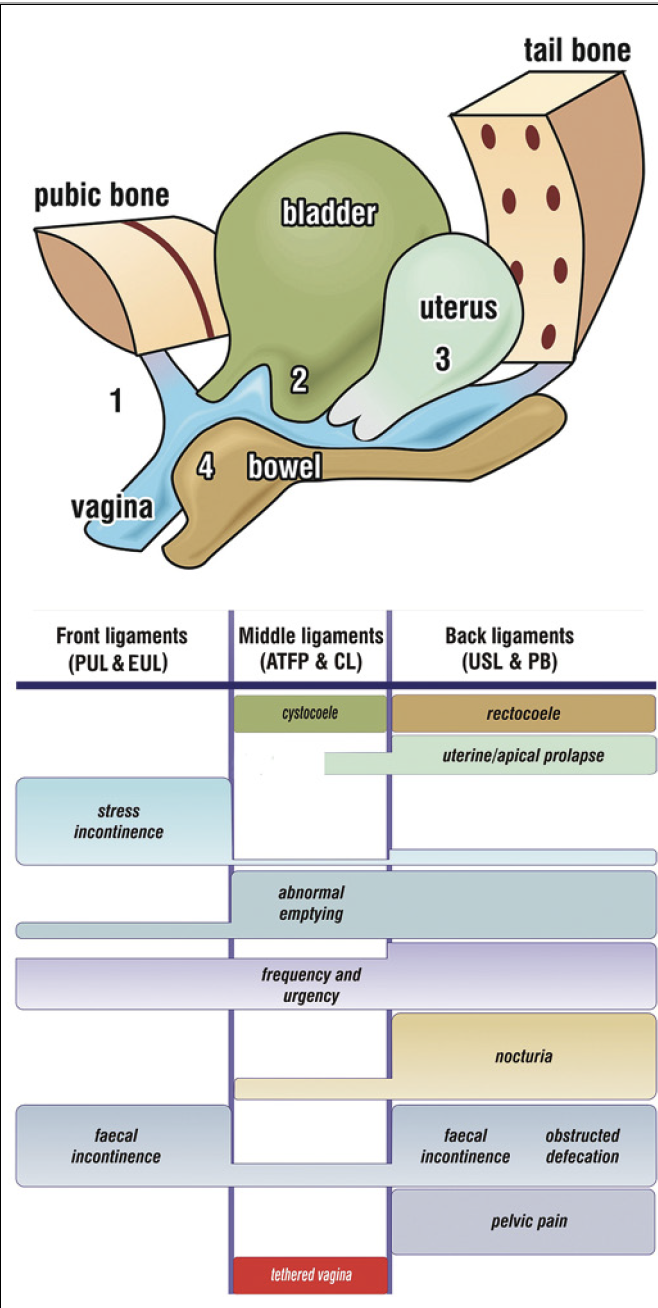
Figure 2. Simplified Pictorial Diagnostic Algorithm. Relates structural damage (prolapse) to symptoms: 1: stress incontinence; 2: cystocele; 3: uterine prolapse; 4: rectocele. The size of the bar gives an approximate indication of the preva-lence (probability) of the symptom. Ligaments which can be repaired are: pubourethral ligament (PUL); CX ring/cardinal ligament (CL); arcus tendineus fascia pelvis (ATFP); uterosacral ligament (USL); perineal body (PB). The main symptom for ‘Tethered vagina syndrome’ is massive urine loss immedi-ately following getting out of bed in the morning. The cause is excessive tightness in the bladder neck area of the vagina. Because pain and urgency have a peripheral neurological origin, even minimal vaginal prolapse may cause major symptoms.
Inclusion Criteria
Patients with symptomatic apical prolapse of 2nd or greater degree, (Pelvic Organ Prolapse Quantification [POPQ] stages 2–4), with at least one OAB symptom (frequency, urgency, nocturia) as defined by the ICS 1.
- Frequency. Emptying the bladder >8 times/day1.
- Nocturia: two or more episodes of waking to micturate at night 1.
- Urge incontinence: at least one episode per day of wetting prior to arrival to the toilet 2.
- Fecal incontinence (non sphincteric): Involuntary loss of either liquid or solid feces more than once per week 11.
- ‘Pelvic pain’ symptoms consistent with ICS de-scriptions 1 and in T11-12 and S2-4 distributions; specifically, lower abdominal, low sacral, vulval, vaginal, paraurethral.
Comorbid medical problems; known causes of fecal incontinence such as external anal sphincter damage; endometriosis; neurological diseases such as multiple sclerosis, proven organ infection, carcinoma or other conditions known to cause pain, blad-der or bowel symptoms.

Figure 1. TFS ligament repair system. 3D sagittal section. Insert TFS adjustable tape and soft tissue anchor set on applicator. The TFS tapes are inserted into all 5 ligaments: pubourethral (PUL), arcus tendineus fascia pelvis (ATFP), cardinal (CL) utero-sacral (USL) and perineal body (PB). This study concerns only the posterior ligaments, cardinal (CL) and uterosacral (USL). The red rectangle defines the TFS implants as used in this study to reinforce CL & USL.
Intervention
All 611 patients underwent a posterior cardinal/ uterosacral TFS operation (Figure 1).
Follow-up and End Point Measurements
At 12 month follow up, a full assessment was made using the self-administered ITSQ questionnaire 11, as well as a vaginal examination.
Criteria for a Positive Response
Criteria for anatomical failure. Any organ prolapse at POPQ stage 2 or beyond. Urinary frequency. Eight or less times per day. Nocturia. Reduction from 2 or more episodes per night to one or nil. Urge incontinence. Zero episodes of wetting prior to arrival to the toilet. Abnormal emptying. Self-assessed 80% improve-ment. The patient was asked to determine, “Over-all, how much improvement do you experience now as compared to your pre-operative symptoms”?
Fecal incontinence: Zero episodes of soiling. Chronic pelvic pain: A self-assessed 80% improve-ment over the baseline symptom at the 12 month visit. “Overall, how much improvement do you expe-rience now as compared to your pre-operative symptoms”? We chose this criterion due to concerns that the visual analogue scale (VAS) could be misleading because of day to day symptom variations.
Surgery
Cardinal (CL) and uterosacral (USL) TFS sling 12. A transverse incision 5 cm wide was made 1 cm above the cervix or hysterectomy scar for the CL and 3–4 cm below the cervix for the USL. Bladder and enterocele were dissected clear. The cardinal and utero-sacral ligaments were identified. A channel was created through the ligaments; the TFS applicator was inserted into the channel, the anchor released, the procedure repeated on the contralateral side and the tape tightened until a resistance was felt.
TFS slings for urinary stress incontinence, cystocele, rectocele and perineal body repairs were performed as required, taking care to avoid any excision of the vagina.
Statistical Analysis
We applied the McNemar x²-tests to test for signifi-cance changes in the symptoms’ incidence-frequency from baseline (preoperative) to the postoperative phase. For each symptom the null hypothesis H0: P(baseline) = P(12 months after surgery) versus H1: P(baseline) ≠ P(12 months after surgery) was tested, with P indicating prevalence or incidence rate. An α = 0.05 was accepted as the nominal level of significance. Because of multiple testing the p-values of the tests were compared to a Bonferroni corrected α (say α*) for keeping the type I error less or equal to 0.05.
Ethics
This was a prospective case study audit. Prior to un-dertaking this study, each unit obtained EC approval for use of the TFS instrument in prolapse and incon-tinence surgery as standard hospital practice. Written consent was obtained from all patients. The principles of the Helsinki Declaration (2008) were followed.
Results
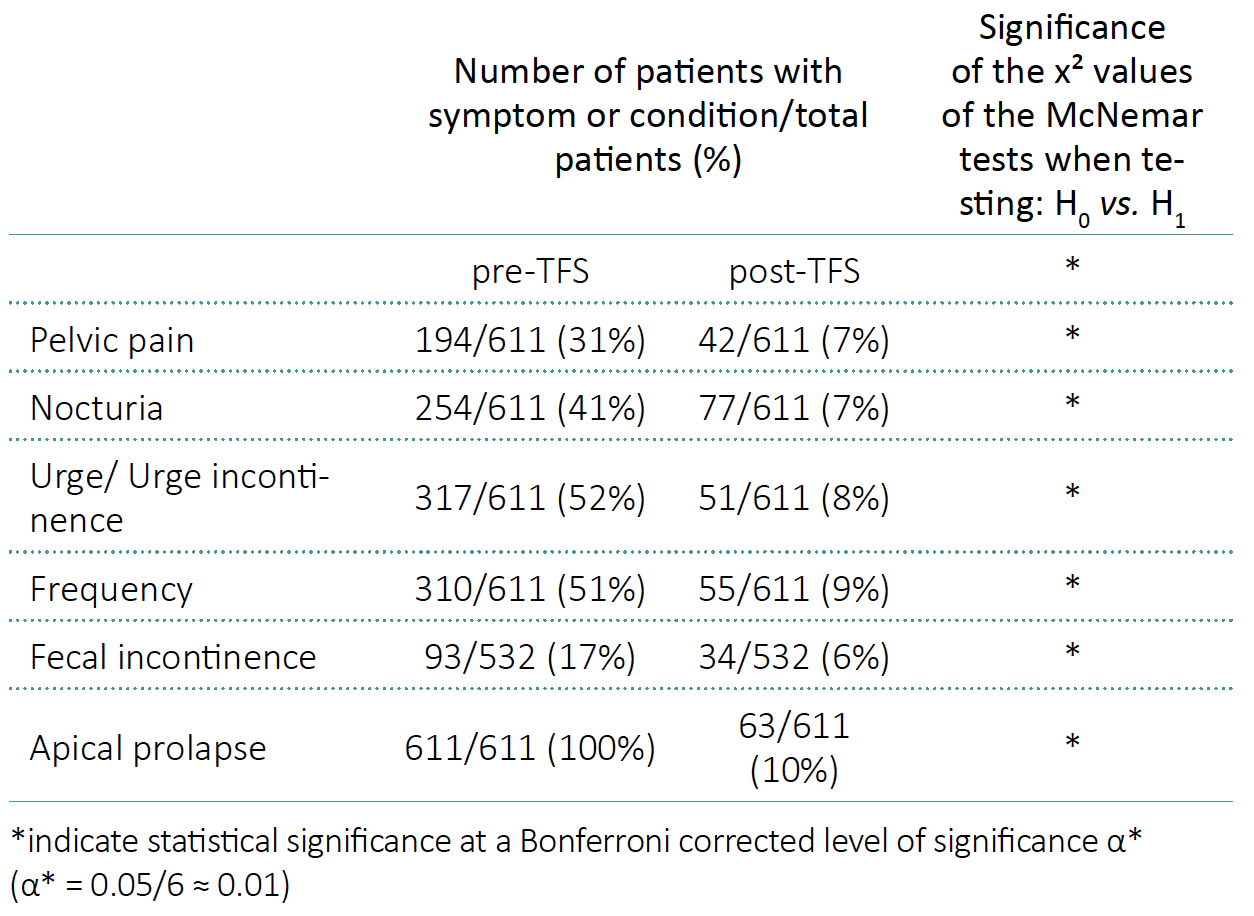
611 patients with apical prolapse and concurrent symptoms were assessed. Only data of patients presenting for a 12 month follow up are presented. Mean age was 69.62 ±13.17 years. Prolapse varied between 2nd degree POPQ (n = 210) and 3rd /4th degree POPQ (n = 390). Of those undergoing TFS surgery 93 (15%) had faecal incontinence, 194 (31%) had pelvic pain, 254 (41%) had nocturia, 317 (52%) had urinary urge incontinence, and 310 (51%) had urinary frequency (Figure 3). There was no correlation between degree of prolapse and symptom severity. Applying McNemar tests, we obtained p-values for each considered symptom, all being less than α*, where α* is a Bonferroni corrected level of significance (Table 1).
Table 1. Symptoms and apical prolapse at baseline and after 12 months in patients operated by the Tissue Fixation System (TFS)
Operative and Peri-Operative Details
The average operating time varied between 12.5 and 23 minutes per TFS tape insertion. The average blood loss per surgery was 21 gm (minimum 4 gm, maxi-mum 136 gm.) There was minimal post-operative pain allowing for early discharge from the hospital. Mean discharge time from hospital was less than 24 hours (0.7–4 days) and return to reasonably normal activities was mean 2.2 days (1–28). Post-operative urinary retention beyond 24 hours occurred in 5 patients: two for 48 hours, one for 4 days and one for 2 weeks.
Complications
There was one rectal mucosal buttonhole injury sustained at initial dissection. It was treated successfully with primary repair. There was one rectal serosal penetration with the prosthesis. It was recognized and removed immediately and successfully without sequelae. Both patients were scarred from previous multiple perineal and posterior compart-ment surgeries. There were three hematomas which required re-admission to the hospital, but these were all settled without further surgery. No transfusion was required. Tape erosion at 12 months varied between centres, from minimum 0% to maximum 3%. There was a 40% association of tape erosion with failed surgery. All erosions were trimmed in the out-patient clinic. None required surgical intervention.
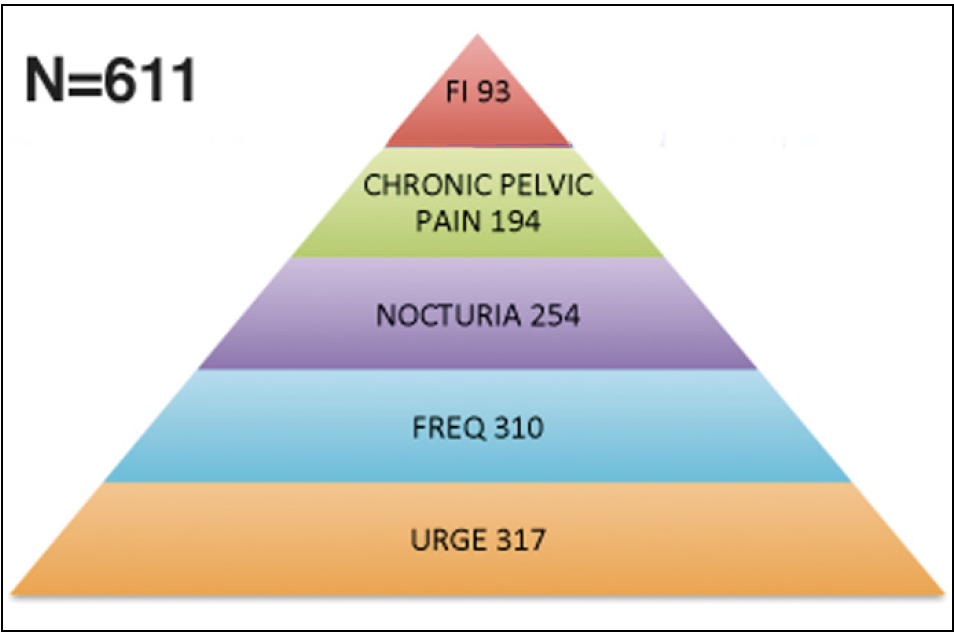
Figure 3. The Pelvic Symptom pyramid – Symptoms occur in predictable groupings. The Relationship of symptoms within this grouping of 611 patients who had cardinal/uterosacral ligament laxity is expressed as a pyramid.
At 12 months Following TFS Surgery
The anatomical recurrence of vaginal prolapse was 63/611 (10%) after 12 months. There was a significant improvement in all symptoms: (cure/improvement in brackets): urge incontinence (85%), frequency (83%), nocturia (68%), fecal incontinence (65%) chronic pelvic pain (77%) (Table 1).
Discussion
Using only small strips of 7 mm wide tape to reinforce cardinal and uterosacral ligaments, we were able to address apical prolapse with an anatomical cure rate of 90% at 12 months without vaginal excision or hysterectomy. Symptoms and large prolapses were successfully corrected without the need and problems of mesh sheets 13 , with minimal complications. The TFS mechanically supports the vagina and bladder base much as joists (ligaments) support a plaster ceiling (vagina). We believe that it is this mechanical support which is crucial in preventing the bladder base stretch receptors prematurely activating the micturition reflex to cause urge, frequency, and nocturia 3,4,5.
We confirmed the ICS statement 1 that urge, frequency, nocturia, chronic pelvic pain and faecal incontinence occur (variably) with each other. For the first time, we were able to quantify the relationship (Figure 3). At 12 months, OAB symptoms were surgically im-proved/cured as follows: urge incontinence (85%), frequency (83%), nocturia (68%). CPP and faecal incontinence were also improved/cured, an impossibility as according to the ICS paradigm 1,2.
The concept of surgical cure of OAB is not new. In the prototype midurethral sling operations which reinforced the pubourethral ligament PUL) 14, of 30 urodynamic stress incontinence (USI) patients, 25 also had urge incontinence (UI) and they reported cure of both stress and urge symptoms post-operatively. These results, cure of ‘mixed incontinence’ were subsequently validated by Rezapour and Ulmsten in a well-controlled urodynamically assisted study where again, only the PUL was repaired 15. However, this is the first large study where the emphasis was on OAB patients without concomitant urinary stress incontinence.
The TFS CL/USL operation compares well with sacrocolpopexy, early discharge, low erosion rates and a quarter of the OR time needed for laparoscopic sacrocolpopexy (SCP) 16, 25–46 minutes compared to 185 minutes, with a more anatomical fixation point, S3-4 rather than the promontory of SCP. The 12 month symptomatic TFS OAB cure also compares well with the 6 month RCT data of anticholinergics and Botox (onabotulinumtoxinA) 17 in 488 patients: at 6 months, complete resolution of urinary urge in-continence was reported by 13% and 27% of the women respectively 17 (P = 0.003), significantly inferior results to this study. No data was given for nocturia or frequency in that RCT study 17 and no data for chronic pelvic pain or faecal incontinence.
Prima facie, the Integral Theory System potentially offers ‘a substantial advancement in understanding causal pathways of disease and treatment’ 18, especially with regard to Women’s Health and the community purse. Moore et al.19 quoted the cost of permanent sacral nerve stimulation at €10,700, dynamic graciloplasty at €12,000 and Artificial Sphincters at €12,000. The cost of the two implants for CL/USL repair was less than 10% of these amounts. The cost of bladder, bowel and pain dysfunctions to the community is well known. Nocturia has a direct cost of EUR 1 billion for Europe 20 just due to fractured hips. Incontinence accounts for more than 50% of admissions to Nursing Homes 21. Perhaps this may be the greatest future benefit of this method, potential reduction in the number of admissions to Nursing Homes.
The strength of this study was its large numbers, 611 patients and its uniqueness, TFS surgical cure of OAB symptoms by repair of apical prolapse and other symptoms such as pain and fecal incontinence; successful application of the ligament-based TFS ligament repair methodology to 70 year old women on a mainly day-care basis; demonstration of high anatomical cure rates without the need for large meshes; the high improvement rates for OAB symptoms gain particular importance, given recent major concerns with anticholinergic drugs 22; Weaknesses. Weakness of this study is the absence of treatment of OAB patients without prolapse, which is not feasible for ethical reasons. The study was observational and not registered. Registration was comparatively rare in 2008 when the study was initially planned.
According to the Integral Theory, the explanation for the cure of OAB symptoms of urge incontinence, frequency, nocturia is the link between loose ligaments and diminished striated muscle force. The directional vectors contract against the anterior and posterior suspensory ligaments (Figure 4). If these are loose, the contractile force weakens 23 (Figure 5). All functions dependent on these muscle vectors, for example, stretching the organs to prevent activation of the micturition reflex, are compromised so that the stretch receptors fire off prematurely. This is perceived as urgency, frequency, and nocturia. The TFS creates a firm suspension system to restore muscle contractility.
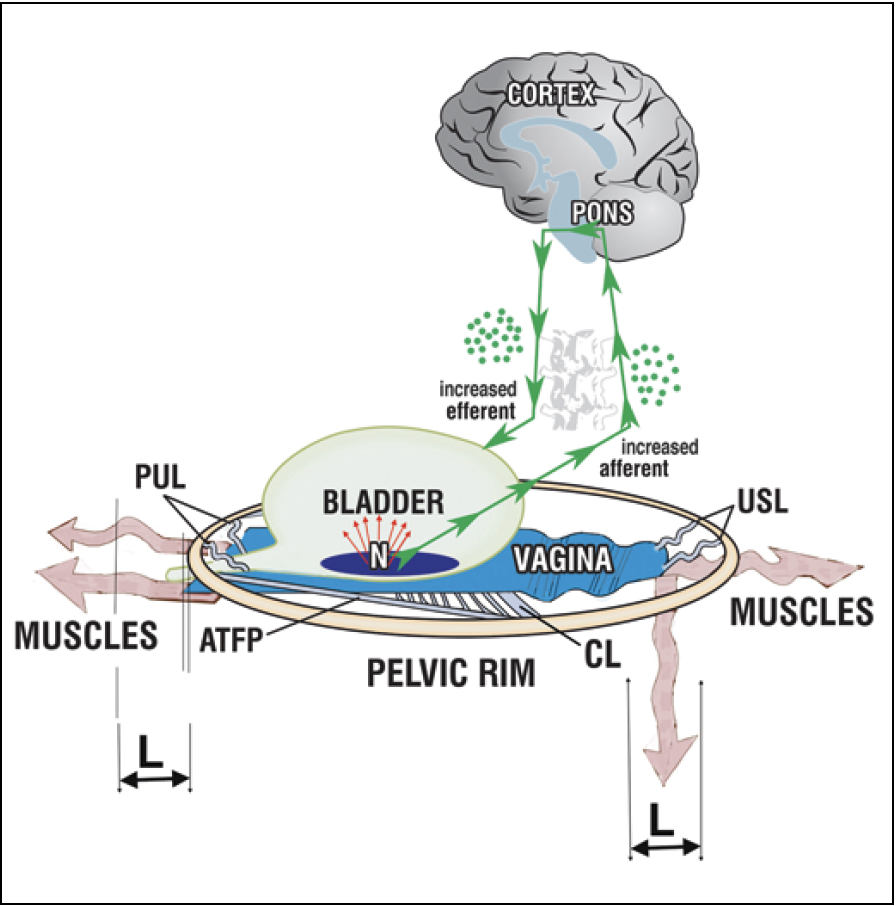
Figure 4. Urge incontinence – a premature activation of a normal micturition reflex. Loose suspensory ligaments (PUL, USL, CL) are unable to suspend the vagina adequately. The muscles (wavy arrows)* which insert into the loose liga-ments lengthen ‘L’; their contractile force weakens; they cannot stretch the vagina sufficiently to support the stretch receptors ‘N’; ‘N’ fire off increased afferent impulses at a low bladder volume and this is perceived by the cortex as urgency. If the afferents are sufficient to activate the micturition reflex, the efferents are activated and the patient may uncontrollably lose urine (‘urge incontinence’). PUL – pubourethral ligament; USL – uterosascral ligament; CL – cardinal ligament. The backward downward arrows are wavy, to emphasize their weakened muscle force.
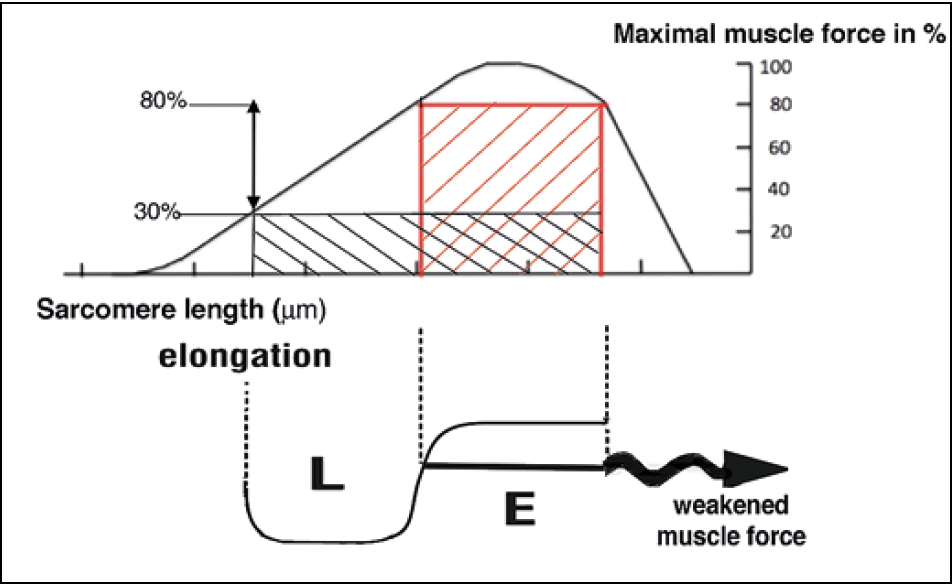
Figure 5. Gordon’s Law. A striated muscle contracts optimally over a short length only, ‘E’, red square. Lengthening the muscle ‘L’, results in a rapid loss of contractile force, black rectangle.
Conclusions
The findings appear to validate the ligament-based Theory behind the TFS surgery described in this work. The minimally invasive nature of the surgery and high improvement rates for dysfunctions such as OAB not presently considered surgically curable, are consistent with Kuhn’s description of a new paradigm, one based on diagnosis and repair of lax ligaments. The Theory also fits Richard Horton’s 2000 Lancet statement of research innovation being a ‘substantial advance in understanding causal pathways of disease and treatment effects across major threats to human health’ 18. This paradigm has the potential to revolutionize clinical practice, improve QOL of ageing women, produce community cost savings, and to reduce the number of Nursing Homes admissions.
Written By: Bernhard Liedl1, Hiromi Inoue2,3, Yuki Sekiguchi3, Max Haverfield4, Peter Richardson5, Alexander Yassourides1, Florian Wagenlehner6
1. Zentrum für Urogenital Chirurgie BBZ, Fachkliniken München AG, Germany
2. Urogynaecology Center, Shonan Kamakura General Hospital, Kamakura, Japan
3. LUNA Pelvic Floor Total Support Clinic, Women’s Clinic LUNA Group, Yokohama, Japan
4. Department of Gynaecology, The Northern Hospital, Melbourne Victoria, Australia
5. Department of Health, Medical and Applied Sciences, University of Central Queensland, Australia University of Central Queensland, Australia
6. Clinic for Urology, Pediatric Urology und Andrology, Justus-Liebig-University Giessen, Germany
Liedl B, Inoue H, Sekiguchi Y, et al. Is overactive bladder in the female surgically curable by ligament repair? Cent European J Urol. 2017; 70: 53-59.
Read An Expert Commentary on this Article
References
1. Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002; 21: 167-178.
2. Andersson K-A, Chapple C, Cardozo L, et al. Pharmacological Treatment of Urinary Incontinence in Incontinence 5th International Consultation Paris Feb. 2012; ISBN 978-9953-493-21-3. Editors Abrams P, Cardozo L, Khoury S, Wein A; pp. 623-728.
3. Petros PE, Ulmsten U. An Integral Theory of female urinary incontinence. Acta Obstet Gynecol Scand Suppl. 153, 1990; 69: 1-79.
4. Petros P, Ulmsten U. Is detrusor instability a prematurely activated (but otherwise normal) micturition reflex? Lancet. 1997; 349: 505
5. Petros PE, Ulmsten U. Bladder instability in women: A premature activation of the micturition reflex. Neurourol Urodyn. 1993; 12: 235-239.
6. Petros PE, Ulmsten U, Papadimitriou J. The Autogenic Neoligament procedure: A technique for planned formation of an artificial neo-ligament. Acta Obstet Gynecol Scand Suppl. 1990; Suppl 153: 43-51.
7. Petros PEP. The Integral System. Cent European J Urol. 2011; 64: 110-119.
8. Neuman M, Lavy Y. Posterior Intra-Vaginal Sling (PIVS) for the treatment of vaginal apex prolapse: medium term results of the 140 operations with a novel procedure. Eur J Obstet Gynecol Reprod Biol. 2008; 140: 230-233.
9. Abendstein B, Petros PE, Richardson PA. Ligamentous repair using the Tissue Fixation System confirms a causal link between damaged suspensory ligaments and urinary and fecal incontinence. J.Pelviperineology. 2008; 27: 114-117.
10. Petros PEP, Richardson PA. The midurethral Tissue Fixation System sling - a ‘micro-method’ for cure of stress incontinence preliminary report. ANZJOG. 2005; 45:372-375.
11. Wagenlehner FM, Fröhlich O, Bschleipfer T, Weidner W, Perletti G. The Integral Theory System Questionnaire: an anatomically directed questionnaire to determine pelvic floor dysfunctions in women. World J Urol. 2014; 32: 769-781.
12. Petros PEP. Creating a gold standard surgical device: scientific discoveries leading to the TVT and beyond: Ulf Ulmsten Memorial Lecture 2014.Int Urogynecol J. 2015; 26: 471-476
13. Maher C, Feiner B, Baessler K, Schmid C. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013; 4: CD004014.
14. Petros PE, Ulmsten U. The combined intravaginal sling and tuck operation. An ambulatory procedure for stress and urge incontinence. Acta Obstet Gynecol Scand. 1990; 69: 53-59.
15. Rezapour M, Ulmsten U. Tension free vaginal tape (TVT) in women with mixed urinary incontinence - A long-term follow-up. Int Urogynecol J Pelvic Floor Dysfunct. 2001; 12: S15-18.
16. Ganatra AM, Rozet F, Salas R, et al. The current status of laparoscopic sacrocolpopexy: a review. Eur Urol. 2009; 55: 1089-1105.
17. Visco AG, Brubaker L, Richter HE, et al. Anticholinergic Therapy vs. Onabotulinu-mtoxinA for Urgency Urinary Incontinence. N Engl J Med. 2012; 367: 1803-1813.
18. Horton R. The Refiguration of Medical Thought. Lancet. 2000; 356: 2-4.
19. Moore KH, Wagner TH, Subak L, De Wachter S, Dudding T, Hu TW. Book Chapter Economics of Urinary & Faecal Incontinence, and Prolapse, Continence In 5th International Consultation on Incontinence, Eds Abrams P, Cardozo L, Khoury S, Wein A, ISBN 978-9953-493-21-3; pp. 1831-1861.
20. Holm-Larsen T. The Economic Impact of Nocturia. Neurourol Urodyn. 2014; 33: S10-14.
21. Morrison A, Levy R. Fraction of nursing home admissions attributable to urinary incontinence.Value Health. 2006; 9: 272-274.
22. Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med. 2015; 175: 401-407.
23. Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966; 184: 170-192.


