BERKELEY, CA (UroToday.com) -
Long-term safety and efficacy of mirabegron, the first beta-3 adrenergic agonist for OAB
Chapple et al. reported on the long term treatment with mirabegron for overactive bladder (OAB) in a randomized, double-blind, active-controlled, phase 3 study published online in European Urology November 6, 2012 ahead of print (NCT00688688). This 12-month study described the long-term efficacy and safety profile of mirabegron, a beta 3 adrenergic agonist developed for the treatment of overactive bladder. Notably, beta-3 adrenergic-receptor agonists such as mirabegron are thought to exert their favorable effects on OAB via stimulation of beta-3 receptors resulting in detrusor smooth muscle relaxation during bladder filling and urinary storage. The design of this study is unique; it’s the first, long term, randomized, active-controlled, safety study conducted in OAB registration trials which includes both safety and efficacy assessments. Importantly, the study was not designed or powered as a head-to-head comparison between the two doses or mirabegron studied (50 and 100 mg once daily) or as compared to an active control, but instead, utilized the active control, tolterodine ER 4mg, to provide clinical context.
The efficacy of mirabegron in treating OAB in this study was evidenced by the numerical improvements in incontinence episodes and micturition frequency observed at the first measured time point of week 4 and sustained throughout the 1-year treatment period at both doses. The magnitude of the effect size tracked, over time, relatively consistently with the active control. Given these results, as well as those garnered from other clinical studies, there appears to be no evidence of tachyphylaxis or tolerance with chronic administration of the beta-3 adrenergic agonist, mirabegron.
In this study, the safety of mirabegron was characterized at both the highest approved therapeutic dose of 50 mg as well as for a supratherapeutic dose of 100 mg over a 1-year treatment period. At the conclusion of this one-year study, the adverse event profile for mirabegron was consistent with 12-week data previously reported by several authors, including Khullar et. al. (European Urology NCT00689104), Nitti et. al. (Journal of Urology NCT00662909) and Herschorn et. al. (Urology NCT00912964).
Table 1: Treatment-emergent adverse events (≥2% in any treatment group) in the mirabegron 1-year long-term safety study 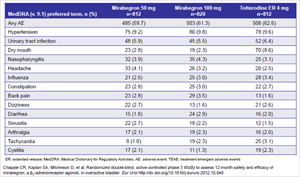
The most common adverse events with mirabegron (>3% incidence in mirabegron-treated patients) reported in this trial include hypertension, urinary tract infection, nasopharyngitis, and headache (Table 1). Dry mouth, a common and adherence-limiting side effect seen with antimuscarinic treatment, was reported in less than 3% of individuals treated with mirabegron.
Given the mechanism of action of mirabegron as a beta-3 adrenergic agonist, potential cardiovascular adverse effects were carefully assessed in this study. A pre-specified protocol definition for the adverse event reporting of hypertension was employed in this trial as a sensitive methodology for detection of such events. The criteria for mandatory reporting of hypertension events included blood pressures exceeding 140/90 mmHg on two consecutive occasions in normotensive patients, blood pressure increases of >20/>10 mmHg on two consecutive occasions in hypertensive patients, or increases in dosage or additions of antihypertensive medications during study conduct. This methodology provided the basis for determining the incidence rates of hypertension observed in this study. Overall, the incidence of hypertension observed was similar across all treatment groups. Vital sign data showed an overall mean increase from baseline in blood pressure of no greater than 1 mmHg and heart rate of approximately 1 bpm in patients receiving mirabegron 50mg. Other safety assessments, including ECG data and laboratory parameters, were clinically unremarkable. The number of major adverse cardiac events in the study was low and similar across treatment groups.
The population enrolled in this clinical study is generalizable to the OAB population seen in practice based on demographics. Of note, approximately 80% of patients enrolled in the long- term safety study were previous participants in the 12-week efficacy studies, whereas approximately 20% were direct enrollers into this study. Importantly, all patients had to qualify on OAB entrance criteria to be eligible for the long-term safety study.
These data provide an important contribution to the OAB literature demonstrating long-term safety and efficacy for mirabegron, a first-in-class new chemical entity for the treatment of OAB. The role of mirabegron in the clinical algorithm for OAB management will declare itself as additional widespread clinical practice and experience is gained. Mirabegron is approved for the treatment of OAB in the U.S. as of June 2012. Mirabegron is also approved for use in Japan and the European Union.
Written by:
Eric Rovner, MD and Nancy Martin, MD, PharmD as part of Beyond the Abstract on UroToday.com. This initiative offers a method of publishing for the professional urology community. Authors are given an opportunity to expand on the circumstances, limitations etc... of their research by referencing the published abstract.
Affliliations: Department of Urology, MUSC, South Carolina and Astellas Scientific and Medical Affairs, Northbrook, IL.
Disclosures: Dr. Rovner is an advisor and consultant for Astellas and Dr. Martin is employed by Astellas.

More Information about Beyond the Abstract


