RIO DE JANEIRO, BRAZIL (UroToday.com) - Presented by Karel Everaert,1 Jennifer Gruenenfelder,2 Heinrich Schulte-Baukloh,3 Steven Guard,4 Yan Zheng,5 and David Sussman6 at the International Continence Society (ICS) 2014 Annual Meeting - October 20 - 24, 2014 - Rio de Janeiro, Brazil
ABSTRACT:
Hypothesis/Aims of Study
OnabotulinumtoxinA 100U significantly reduces urinary incontinence (UI) and improves quality of life (QOL) as demonstrated in two phase 3, randomised, placebo-controlled trials of patients with idiopathic overactive bladder (OAB) who were inadequately managed by ≥ 1 anticholinergic therapy. OnabotulinumtoxinA was well tolerated, with adverse events primarily localised to the urinary tract. Initiation of clean intermittent catheterisation (CIC) and urinary tract infection (UTI) occurred at a higher frequency in OAB patients following treatment with onabotulinumtoxinA compared with placebo. Therefore, the impact of the use of CIC, and the presence of UTI on the treatment response to onabotulinumtoxinA 100U were analysed in OAB patients with UI. In addition, patient baseline demographics and disease characteristics were analysed for possible trends associated with CIC use and UTI status. Duration of CIC, and time to onset of UTI were also determined.
Study Design, Materials and Methods
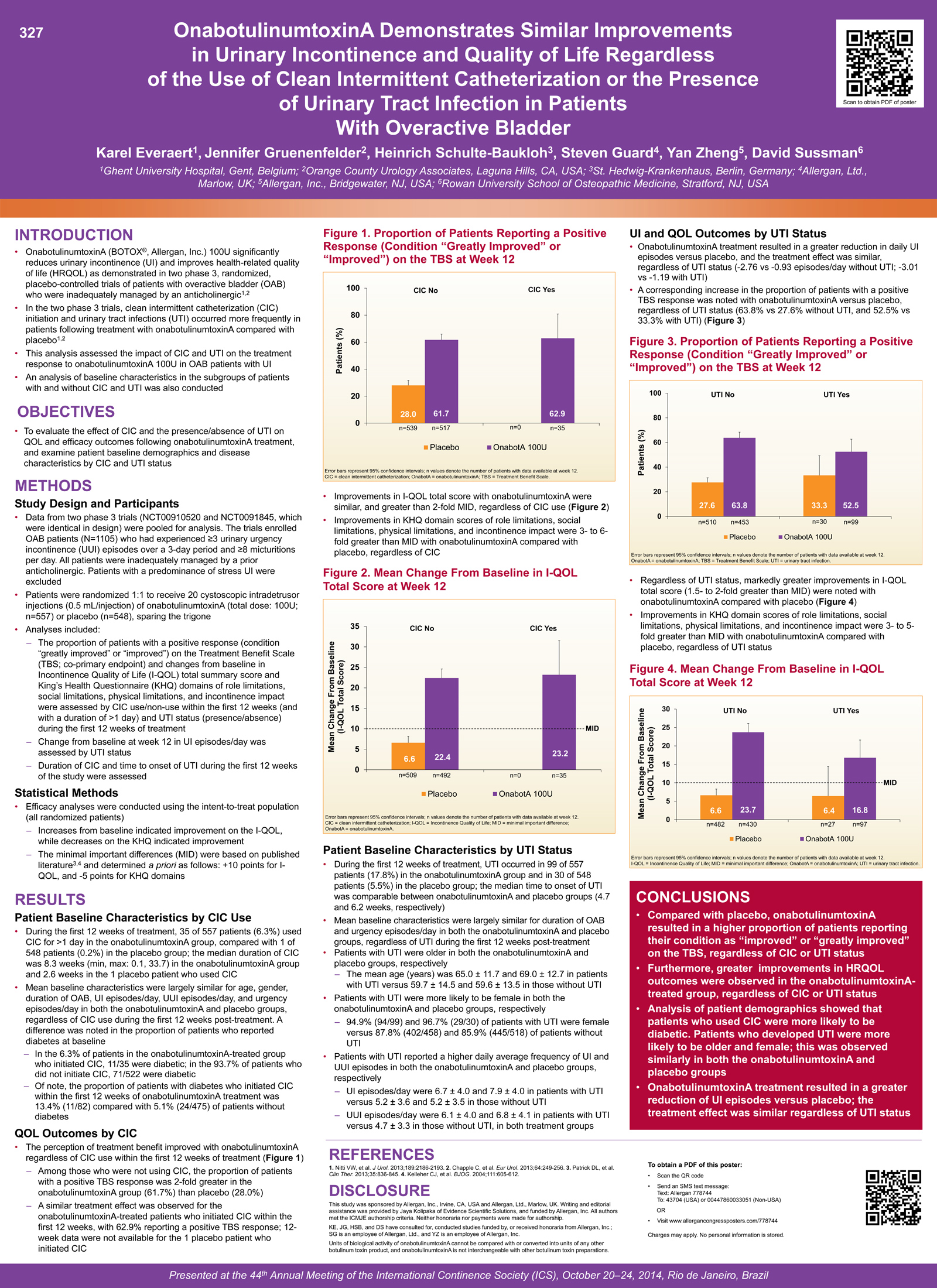 Two phase 3 trials (NCT00910520 and NCT0091845) enrolled OAB patients (N=1105) who had experienced ≥ 3 urinary urgency incontinence (UUI) episodes over a 3-day period and ≥ 8 micturitions per day. All patients were inadequately managed by ≥ 1 prior anticholinergic. Patients with a predominance of stress UI were excluded. Patients were randomised 1:1 to receive 20 cystoscopic intradetrusor injections (0.5 mL/injection) of onabotulinumtoxinA (total dose: 100U; n=557) or placebo (n=548), sparing the trigone. Data from the two phase 3 trials, identical in design, were pooled for analysis. The following parameters were assessed by CIC use/non-use within the first 12 weeks (duration > 1 day) and UTI status (presence/absence) during the first 12 weeks of treatment: baseline demographics and disease characteristics, the proportion of patients with a positive response (condition ‘greatly improved’ or ‘improved’) on the treatment benefit scale (TBS), and changes from baseline in incontinence-quality of life (I-QOL) total score and King’s Health Questionnaire (KHQ) domains of role limitation, social limitation, physical limitation and incontinence impact. Change from baseline at week 12 in UI episodes/day was assessed by UTI status. Duration of CIC (for patients who catheterised during the first 12 weeks) and time to onset of UTI (for patients who has a UTI during the first 12 weeks) was determined. The minimal important differences (MIDs) were based on published literature and determined a priori as follows: +10 points for I-QOL, and -5 points for KHQ domains.
Two phase 3 trials (NCT00910520 and NCT0091845) enrolled OAB patients (N=1105) who had experienced ≥ 3 urinary urgency incontinence (UUI) episodes over a 3-day period and ≥ 8 micturitions per day. All patients were inadequately managed by ≥ 1 prior anticholinergic. Patients with a predominance of stress UI were excluded. Patients were randomised 1:1 to receive 20 cystoscopic intradetrusor injections (0.5 mL/injection) of onabotulinumtoxinA (total dose: 100U; n=557) or placebo (n=548), sparing the trigone. Data from the two phase 3 trials, identical in design, were pooled for analysis. The following parameters were assessed by CIC use/non-use within the first 12 weeks (duration > 1 day) and UTI status (presence/absence) during the first 12 weeks of treatment: baseline demographics and disease characteristics, the proportion of patients with a positive response (condition ‘greatly improved’ or ‘improved’) on the treatment benefit scale (TBS), and changes from baseline in incontinence-quality of life (I-QOL) total score and King’s Health Questionnaire (KHQ) domains of role limitation, social limitation, physical limitation and incontinence impact. Change from baseline at week 12 in UI episodes/day was assessed by UTI status. Duration of CIC (for patients who catheterised during the first 12 weeks) and time to onset of UTI (for patients who has a UTI during the first 12 weeks) was determined. The minimal important differences (MIDs) were based on published literature and determined a priori as follows: +10 points for I-QOL, and -5 points for KHQ domains.
Results
During the first 12 weeks of treatment, 35 of 557 (6.3%) patients catheterised for >1 day in the onabotulinumtoxinA group, compared with 1 of 548 (0.2%) patients in the placebo group. Mean baseline characteristics were largely similar in both onabotulinumtoxinA- and placebo-treated patients in the subgroups by CIC use, with the exception of a few notable trends: the proportion of onabotulinumtoxinA-treated patients who reported having diabetes at baseline was higher in the subgroup with CIC compared with patients in the subgroup without CIC (31.4% [11/35] vs 13.6% [71/522], respectively). In the 35 patients who initiated CIC during the first 12 weeks after onabotulinumtoxinA treatment, the median duration was 8.3 weeks (min; max: 0.1; 33.7). The 1 placebo patient who catheterised during the first 12 weeks had a duration of 2.6 weeks. The perception of treatment benefit improved with onabotulinumtoxinA regardless of CIC. At week 12, a positive TBS response was observed in 61.7% of patients (319/517) in the onabotulinumtoxinA group who were not using CIC, compared with 62.9% of patients (22/35) who initiated CIC. OnabotulinumtoxinA treatment resulted in similar improvements in QOL measures regardless of CIC. Clinically relevant increases in I-QOL total score (>2-fold MID) were seen at week 12 in patients without CIC (mean increase, 22.4 points) and with CIC (mean increase, 23.2 points). Improvements in KHQ domain scores of role limitations, social limitations, physical limitations, and incontinence impact were also similar regardless of CIC, with clinically relevant improvements (3 to 6-fold >MID) in all four KHQ domains. During the first 12 weeks, UTI occurred in 99/557 (17.8%) patients in the onabotulinumtoxinA group and in 30/548 (5.5%) patients in the placebo group. Baseline characteristics were largely similar in both the onabotulinumtoxinA and placebo-treated patients across the subgroups by UTI status, although an association was observed between age and gender and the presence of UTI: patients who were older and female were more likely to have UTI in both onabotulinumtoxinA and placebo treated groups. There was also an association between the presence of UTI and the number of UI and UUI episodes at baseline; patients with UTI reported a higher daily average frequency of UI and UUI episodes/day than patients without UTI. The median time to onset of UTI was comparable between the onabotulinumtoxinA (4.7 weeks) and placebo groups (6.2 weeks). OnabotulinumtoxinA treatment resulted in a greater reduction in daily UI episodes versus placebo, and the treatment effect at week 12 was similar regardless of UTI status (-2.76 vs -0.93 episodes/day without UTI; -3.01 vs -1.19 with UTI). A corresponding increase in the proportion of patients with a positive TBS response was noted with onabotulinumtoxinA versus placebo at week 12, regardless of UTI status (63.8% vs 27.6% without UTI, and 52.5% vs 33.3% with UTI). Improvements in I-QOL total score were similar regardless of UTI status (mean increases of 23.7 vs 6.6 points without UTI, and mean increases of 16.8 vs 6.4 points with UTI). In contrast to the placebo group, clinically relevant improvements (1.5- to 2-fold >MID) were noted with onabotulinumtoxinA in I-QOL total score, regardless of UTI status. Clinically relevant improvements (3- to 6-fold >MID) were also observed in all four KHQ domain scores following onabotulinumtoxinA treatment that were similar regardless of UTI status.
Interpretation of Results
The results of these subgroup analyses suggest that there may be an association between baseline age, gender, frequency of daily UI, and UUI episodes, and the presence of UTI in both onabotulinumtoxinA and placebo groups. In the onabotulinumtoxinA group, the results suggest that there may be an association between CIC use and diabetes at baseline.The median duration of CIC following onabotulimumtoxinA treatment was 8.3 weeks. The time to onset of UTI was similar at 5-6 weeks in both the onabotulinumtoxinA and placebo groups. Importantly, the QOL improvements with onabotulinumtoxinA were clinically meaningful and similar, regardless of CIC use or UTI status. Daily UI episodes were reduced with onabotulinumtoxinA regardless of UTI status. These improvements were reflected in corresponding increases in treatment benefit, with 53-64% of the OAB patients reporting their condition as greatly improved or improved with onabotulinumtoxinA regardless of CIC use or UTI status.
Concluding Message
OnabotulinumtoxinA increased the positive perception of treatment benefit and provided clinically relevant improvements in QOL outcomes, regardless of CIC use or UTI status, in OAB patients who were inadequately managed by ≥ 1 anticholinergic therapy. Furthermore, onabotulinumtoxinA treatment resulted in similar reductions in daily incontinence episodes regardless of UTI status.
Disclosures
Funding: Allergan, Inc. Clinical Trial: Yes Registration Number: NCT00910520 and NCT0091845 RCT: Yes Subjects: HUMAN Ethics Committee: Institutional Review Board Helsinki: Yes Informed Consent: Yes
1Ghent University Hospital, Gent, Belgium; 2Orange County Urology Associates, Laguna Hills, CA, USA; 3St. Hedwig-Krankenhaus, Berlin, Germany; 4Allergan, Ltd., Marlow, UK; 5Allergan, Inc., Bridgewater, NJ, USA; 5Rowan University School of Osteopathic Medicine, Stratford, NJ, USA


