RIO DE JANEIRO, BRAZIL (UroToday.com) - Presented by Errando Smet,1 Garmendia Larrea,2 Jiménez Cidre,3 Clara Loveman,4 Kristin Khalaf,5 Javier Aracil,6 Sanja Stanisic,7 and Johanna Lister8 at the International Continence Society (ICS) 2014 Annual Meeting - October 20 - 24, 2014 - Rio de Janeiro, Brazil
ABSTRACT:
Hypothesis/Aims of Study
BOTOX® (onabotulinumtoxinA) is a newly licensed treatment option for overactive bladder (OAB) for patients who are inadequately managed with anticholinergic medications. Patients comprising the target population for onabotulinumtoxinA have already failed on pharmacotherapy but have not yet undergone more invasive procedures. For these patients, the treatment choices are to receive Best Supportive Care (BSC) – consisting of a combination of behavioural therapy, incontinence pads, occasionally catheters and for a proportion of patients, anticholinergic therapy – or onabotulinumtoxinA. The objective of this study was to evaluate the cost-effectiveness of onabotulinumtoxinA versus BSC from the perspective of the Spanish National Health System (SNHS).
Study Design, Materials and Methods
 A 10-year Markov state-transition model, divided into 3-month cycles, was developed to reflect this patient population from the SNHS perspective. In the model, patients received either onabotulinumtoxinA or BSC. Health states were determined by daily number of UI episodes (UIE) (Figure 1). Rate of urodynamic assessment was assumed to be equivalent in both arms. Sacral nerve stimulation (SNS) was introduced as a downstream therapy in the model as some patients will consider the use of this treatment after using onabotulinumtoxinA or BSC. In a scenario analysis, onabotulinumtoxinA was compared directly with SNS. Unit costs (in EUR) were derived from public price lists and tariffs from the SNHS. Effectiveness was measured in terms of quality adjusted life years (QALYs). Patient baseline characteristics, treatment effects and health state utility values were taken from onabotulinumtoxinA phase 3 trials and a long-term open label extension trial (EMBARK studies). Health state cut-offs were based on results from EMBARK. EQ-5D utility values were obtained indirectly from the Incontinence Quality of Life Questionnaire through a published mapping algorithm. The primary outcome measure, cost per QALY gained, was calculated by estimating the mean incremental costs and QALYs for each group after discounting costs and QALYs at 3%. Two and 5-year time horizons were used in the scenario analyses. Univariate and probabilistic sensitivity analyses (SA) were conducted.
A 10-year Markov state-transition model, divided into 3-month cycles, was developed to reflect this patient population from the SNHS perspective. In the model, patients received either onabotulinumtoxinA or BSC. Health states were determined by daily number of UI episodes (UIE) (Figure 1). Rate of urodynamic assessment was assumed to be equivalent in both arms. Sacral nerve stimulation (SNS) was introduced as a downstream therapy in the model as some patients will consider the use of this treatment after using onabotulinumtoxinA or BSC. In a scenario analysis, onabotulinumtoxinA was compared directly with SNS. Unit costs (in EUR) were derived from public price lists and tariffs from the SNHS. Effectiveness was measured in terms of quality adjusted life years (QALYs). Patient baseline characteristics, treatment effects and health state utility values were taken from onabotulinumtoxinA phase 3 trials and a long-term open label extension trial (EMBARK studies). Health state cut-offs were based on results from EMBARK. EQ-5D utility values were obtained indirectly from the Incontinence Quality of Life Questionnaire through a published mapping algorithm. The primary outcome measure, cost per QALY gained, was calculated by estimating the mean incremental costs and QALYs for each group after discounting costs and QALYs at 3%. Two and 5-year time horizons were used in the scenario analyses. Univariate and probabilistic sensitivity analyses (SA) were conducted.
Results
Over a 10-year time horizon, total costs were €13,848 for onabotulinumtoxinA and €14,335 for BSC; total QALYs gained were 7.219 and 6.977, respectively, showing onabotulinumtoxinA to be a more effective and less costly (i.e., dominant) treatment option.
In the direct comparison analysis with SNS, onabotulinumtoxinA was the economically dominant treatment option (total costs: €13,848 for onabotulinumtoxinA and €20,777 for SNS; total QALYs gained: 7.219 and 6.906, respectively).
Reducing the time horizon to two and 5 years yielded an incremental cost-effectiveness ratio (ICER) of €2,495/QALY and €827/QALY, respectively. Results remained dominant under the majority of univariate SA. Probabilistic SA showed approximately 80% probability that the ICER will fall below €20,000.
Interpretation of Results
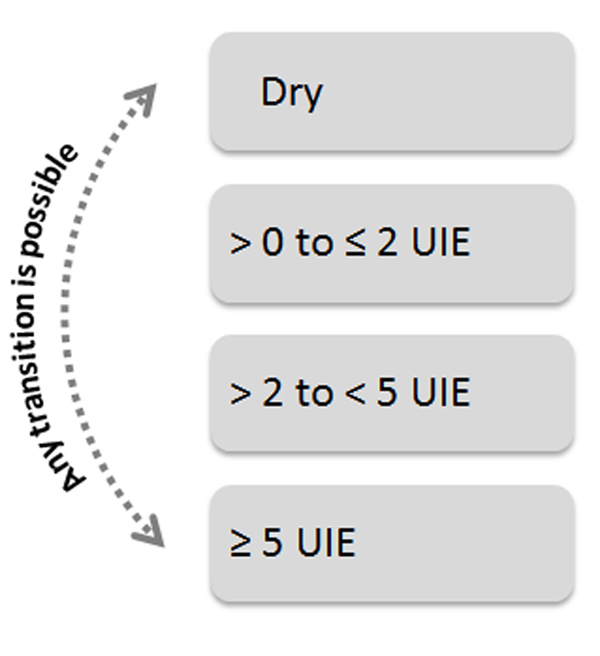 |
| Figure. 1 Disease progression in the Markov state-transition model |
Results from this cost-effectiveness analysis suggest that the use of onabotulinumtoxinA in the treatment of patients who are inadequately managed with anticholinergic medication is a cost-effective option compared with BSC alone from the Spanish perspective. OnabotulinumtoxinA results in lower overall costs and greater QALY gain under most scenarios, attributable to relief of UI symptoms, subsequent decrease in resource utilization and resulting QALY gain.
Concluding Message
For patients with OAB who are not adequately managed with anticholinergics, onabotulinumtoxinA is a cost-effective use of resources in Spain.
Disclosures
Funding: Allergan Limited, UK; Allergan, Inc., USA Clinical Trial: No Subjects: NONE
1Fundació Puigvert, Barcelona; Spain; 2Hospital Donostia, Gipuzkoa, Spain; 3Hospital Universitario Ramón y Cajal, Madrid, Spain; 4Allergan Holdings Ltd, Marlow, UK; 5Allergan Inc., Irvine CA, USA; 6Allergan España, Madrid, Spain; 7LA-SER ANALYTICA, Milano, Italy; 8LA-SER ANALYTICA, Lörrach, Germany


