RIO DE JANEIRO, BRAZIL (UroToday.com) - Presented by J. Seth,a K. Tudor,a M. Liechti,a J. Ochulor,a G. Gonazales,a C. Haslam,a Z. Fox,b M. Pakzad,a S. Elneil,a and J. Panickera at the International Continence Society (ICS) 2014 Annual Meeting - October 20 - 24, 2014 - Rio de Janeiro, Brazil
ABSTRACT:
Hypothesis/Aims of Study
Percutaneous Tibial Nerve Stimulation (PTNS) is a minimally invasive neuromodulation technique for treating overactive bladder (OAB) symptoms. The aim of this study was to assess safety and efficacy in neurological patients being referred to a tertiary centre.
 Study Design, Materials and Methods
Study Design, Materials and Methods
This is a prospective evaluation over 18 months of patients who found first line treatments intolerable or ineffective. PTNS treatment was administered in a weekly course of 30 minute stimulations, using UrgentPC, from Uroplasty, for a total of 12 weeks. Symptoms were objectively assessed using the ICIQOAB and ICIQLUTS-QoL questionnaires and bladder diaries at baseline and after 12 weeks of treatment. Patients opting to continue treatment after 12 weeks returned for top-up session.
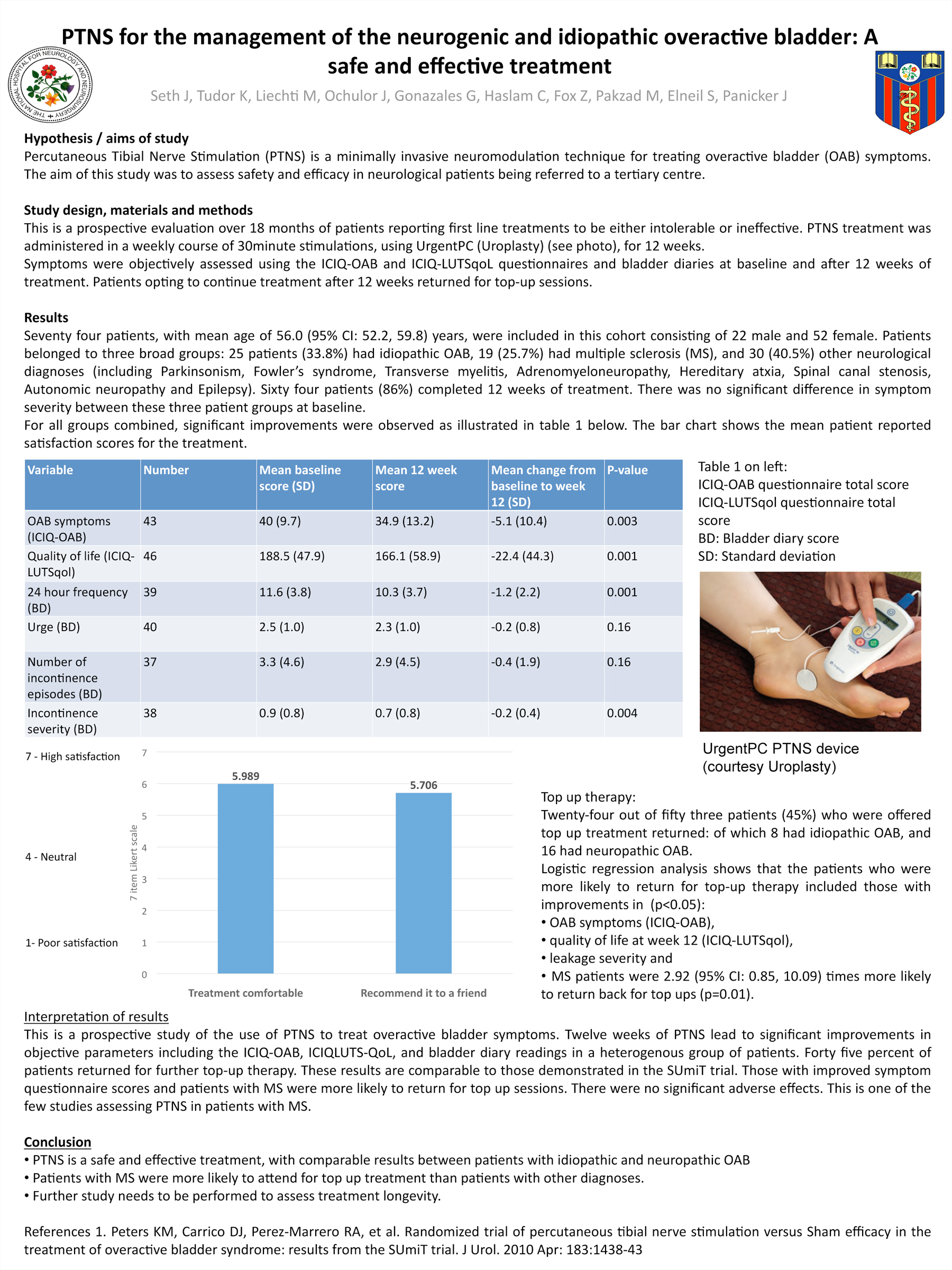
Results
Seventy four patients, with mean age of 56.0 (95% CI: 52.2, 59.8) years, were included in this cohort consisted of 22 males and 52 female patients. These patients belonged to three broad groups: twenty five patients (33.8%) had idiopathic OAB, 19 (25.7%) had multiple sclerosis (MS), and 30 (40.5%) other neurological diagnoses (including Parkinsonism, Fowler’s syndrome, Transverse myelitis, Adrenomyeloneuropathy, Hereditary atxia, Spinal canal stenosis, Autonomic neuropathy and Epilepsy). Sixty four patients (86%) completed 12 weeks of treatment. There was no significant difference in symptom severity between these three patient groups at baseline.
For all groups combined, significant improvements were noted with median decreases in total ICIQ-OAB scores of -3 (IQR: -11.5, 5) (p=0.01), total ICIQLUTS-QoL scores -16 (IQR: -57, 6.5) (p=0.004), and reduced bladder diary parameters of 24-hour urinary frequency -1.67 (IQR: -3.0, 0.33) (p=0.002), number of incontinence episodes -0.0 (IQR: -1, 0) (p=0.01) and severity 0 (IQR: -0.33, 0) (p=0.007) of incontinence episodes.
There were no serious adverse effects of treatment. Twenty-four out of fifty three patients (45%) who were offered top up treatment returned: of which 8 had idiopathic OAB, and 16 had neuropathic OAB, with a mean frequency of 1.3 top ups/month. The mean top-up interval was 44.4 days (7-155 days). A logistic regression analysis shows that the patients who reported improvements in OAB symptoms, leakage severity and quality of life at week 12, as well as patients with MS, were more likely to return for top-up sessions (p<0.05). MS patients were 2.92 (95% CI: 0.85, 10.09) times more likely to return back for top ups (p=0.01) compared to those with other neurological diagnoses who were 0.43 (95% CI: 0.12, 1.52) less likely to return for top-ups.
Interpretation of Results
This is a prospective study of the use of PTNS to treat overactive bladder symptoms. Twelve weeks of PTNS lead to significant improvements in objective parameters including the ICIQ-OAB, ICIQLUTS-QoL, and bladder diary readings in a heterogenous group of patients. There were no significant adverse effects. Patients with improved symptom questionnaire scores and patients with MS were more likely to return for top up sessions.
Concluding Message
PTNS is a safe and effective treatment for the overactive bladder. Patients with MS were more likely to attend for top up treatment than patients with other diagnoses. Further study needs to be performed to assess treatment longevity.
References
- Peters KM, Carrico DJ, Perez-Marrero RA, et al. Randomized trial of percutaneous tibial nerve stimulation versus Sham efficacy in the treatment of overactive bladder syndrome: results from the SUmiT trial. J Urol. 2010 Apr: 183:1438-43
Disclosures
Funding: This study was led by the department of Uro-Neurology, National Hospital for Neurology and Neurosurgery, Queen Square Clinical Trial: No Subjects: HUMAN Ethics not Req'd: This is a prospective case series using an approved treatment in a unique group of patients Helsinki: Yes Informed Consent: Yes
aDepartment of Uro-neurology, National Hospital for Neurology and Neurosurgery, Queen Square, bDepartment of Statistics, Institute of Neurology, Queen Square


