Our recent study analyzed 384 patients with nonmetastatic SCCB included in the U.S. Surveillance, Epidemiology and End Results (SEER) database, in order to investigate the impact of chemotherapy, surgery, and radiotherapy on overall survival.2 Chemotherapy (neo-adjuvant, perioperative or adjuvant) was associated with a better outcome. In addition, patients with T2 disease treated with chemosurgery showed better survival compared to those who underwent chemoradiation.
Our analysis has several intrinsic limits and this last observation might be related to confounder factors that cannot be adjusted in multivariable analysis or might reflect the possible clinical downstaging of patients who received radiotherapy. However, our data might also support the assumption that SCCB exhibits different tumor biology and clinical behavior compared to SCLC. In fact, concomitant chemoradiotherapy plus prophylactic cranial irradiation, rather than chemo-surgery, is usually recommended as the best treatment option for SCLC, based on the notion that this neoplasm is highly aggressive and disseminates early3.
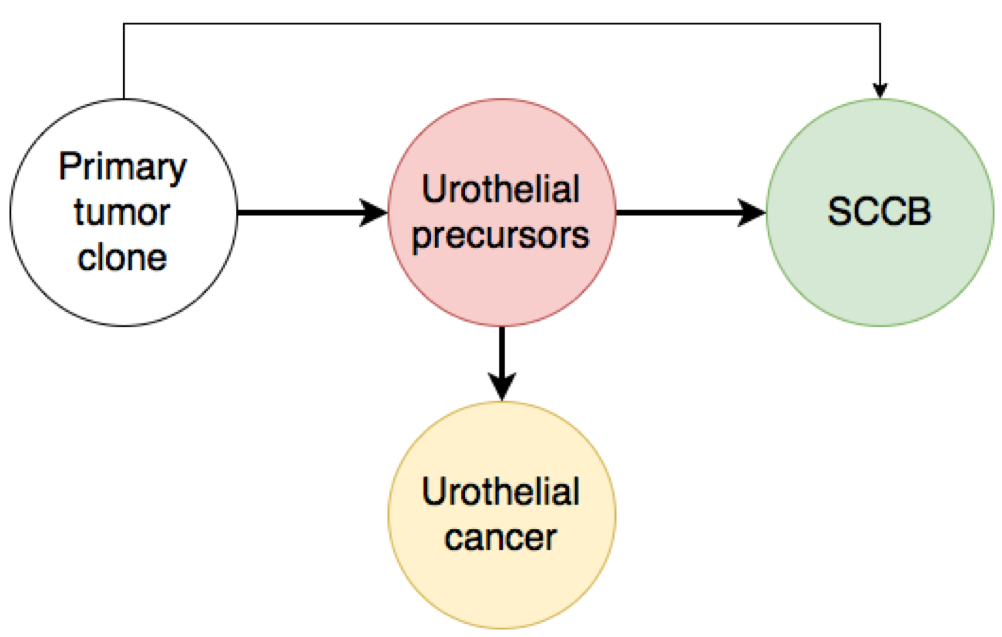
Shen and colleagues have recently performed deep molecular profiling of SCCB.4 These neoplasms harbor frequent genetic aberrations, involving TP53, RB1, PIK3CA, ERCC2, ARID1A, and EP300, which have been implicated in neuroendocrine differentiation and urothelial bladder cancer.4 These findings are consistent with those of previous studies and suggest that the small cell component is often the result of divergent differentiation from a single common progenitor.5-7 Therefore, lineage switching, rather than an independent progression, is probably the predominant mechanism of development of SCCB.8 A similar divergent clonal evolution has been widely described in pretreated castration-resistant neuroendocrine prostate cancer.9 However, lineage switching may also occur in the context of de novo disease without the selective pressure of treatments.
Although all small cell tumors share organ-independent common molecular and pathologic features, the biology of SCCB still probably recalls their primary origin. SCCB is aggressive, undifferentiated neoplasms, which retain site-specific alterations and show neuroendocrine features. Response to chemotherapy, surgery or radiotherapy is dependent on the driving aberrations that occur in each specific framework. Platinum-based chemotherapy is active, but SCCB should not be invariably associated with SCLC. For example, the uninspiring results obtained with immune checkpoint inhibitors for the latter should not discourage from investigating the activity of immunotherapy for the systemic treatment of SCBC.10,11
In the lack of a deep personalized molecular characterization, a multimodality approach focused on the site of origin is probably the best treatment choice for patients with SCCB. “The apple doesn’t fall far from the tree”: until otherwise proven, genitourinary imprinting guides the evolution of these small cell tumors.
Fig.1. Origin of SCCB. Several studies suggest that lineage switching from urothelial precursors is the predominant mechanism of development of SCCB.

Written by: Carlo Cattrini, MD, Elisa Zanardi, Alessandra Rubagotti, PhD, Francesco Boccardo, MD, Academic Unit of Medical Oncology, San Martino Polyclinic Hospital, Genoa, Italy
References:
1. Kouba EJ, Cheng L. Understanding the Genetic Landscape of Small Cell Carcinoma of the Urinary Bladder and Implications for Diagnosis, Prognosis, and Treatment: A Review. JAMA oncology 2017; 3(11): 1570-8.
2. Cattrini C, Cerbone L, Rubagotti A, et al. Prognostic Variables in Patients With Non-metastatic Small-cell Neuroendocrine Carcinoma of the Bladder: A Population-Based Study. Clinical genitourinary cancer 2019.
3. Barnes H, See K, Barnett S, Manser R. Surgery for limited-stage small-cell lung cancer. The Cochrane database of systematic reviews 2017; 4: Cd011917.
4. Shen P, Jing Y, Zhang R, et al. Comprehensive genomic profiling of neuroendocrine bladder cancer pinpoints molecular origin and potential therapeutics. Oncogene 2018.
5. Chang MT, Penson A, Desai NB, et al. Small-Cell Carcinomas of the Bladder and Lung Are Characterized by a Convergent but Distinct Pathogenesis. Clinical cancer research : an official journal of the American Association for Cancer Research 2018; 24(8): 1965-73.
6. Cheng L, Jones TD, McCarthy RP, et al. Molecular genetic evidence for a common clonal origin of urinary bladder small cell carcinoma and coexisting urothelial carcinoma. The American journal of pathology 2005; 166(5): 1533-9.
7. Priemer DS, Wang M, Zhang S, et al. Small-cell Carcinomas of the Urinary Bladder and Prostate: TERT Promoter Mutation Status Differentiates Sites of Malignancy and Provides Evidence of Common Clonality Between Small-cell Carcinoma of the Urinary Bladder and Urothelial Carcinoma. European urology focus 2017.
8. Rickman DS, Beltran H, Demichelis F, Rubin MA. Biology and evolution of poorly differentiated neuroendocrine tumors. Nature medicine 2017; 23(6): 1-10.
9. Beltran H, Prandi D, Mosquera JM, et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nature medicine 2016; 22(3): 298-305.
10. Wilde L, Ali SM, Solomides CC, Ross JS, Trabulsi E, Hoffman-Censits J. Response to Pembrolizumab in a Patient With Chemotherapy Refractory Bladder Cancer With Small Cell Variant Histology: A Case Report and Review of the Literature. Clinical genitourinary cancer 2017; 15(3): e521-e4.
11. Facchinetti F, Marabelle A, Rossi G, Soria JC, Besse B, Tiseo M. Moving Immune Checkpoint Blockade in Thoracic Tumors beyond NSCLC. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2016; 11(11): 1819-36.
Read the Abstract


