What was found: After a median of 33 months of follow-up, OS was analyzed in the ITT population and in subgroups based on PD-L1 status (IC2/3 and IC1/2/3) or investigators’ chemotherapy choice (taxanes or vinflunine). Overall, the OS rates were higher in the atezolizumab- than chemotherapy-treated patients at 24 months (23% vs 13%) and 30 months (18% vs 10%), including across PD-L1–defined subgroups (Table 1).5 These findings were generally consistent with those in the primary analysis.4
Table 1. Long-term survival outcomes in the IMvigor211 ITT population and PD-L1 subgroups

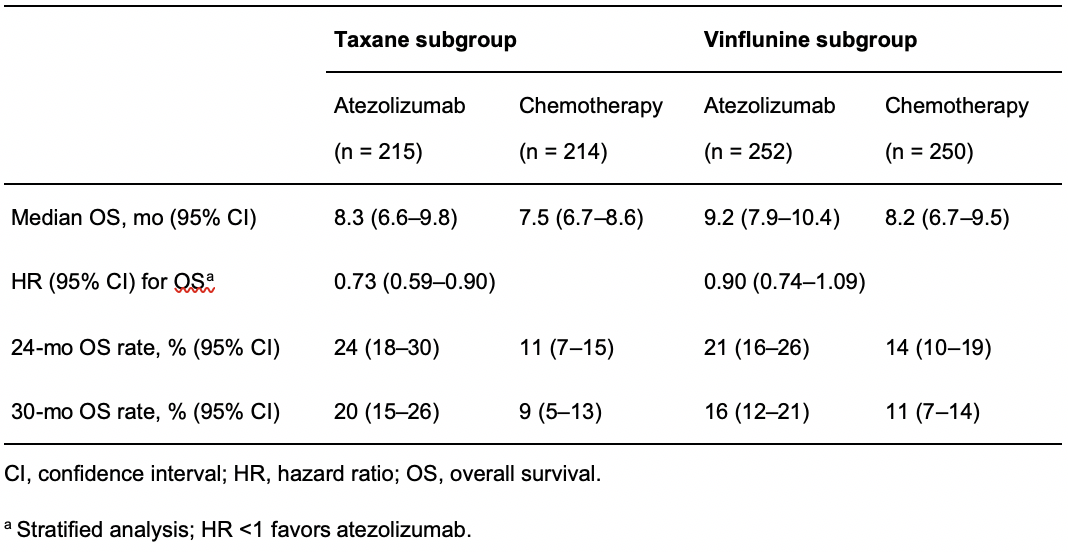
The long-term OS rates were also numerically higher in patients receiving atezolizumab than in those receiving vinflunine or taxanes (Table 2).
Table 2. Survival outcomes in the chemotherapy subgroups
The favorable safety profile of atezolizumab compared with chemotherapy was maintained in this long-term follow-up analysis. Atezolizumab-treated patients experienced fewer grade 3 or 4 treatment-related adverse events (AEs; 22% vs 43%) and fewer AEs leading to treatment discontinuation (9% vs 18%) than those treated with chemotherapy.5 However, patients receiving atezolizumab experienced more AEs of special interest (AESIs; 35% vs 20% with chemotherapy); the most common was immune-related rash (21% vs 12%). Most AESIs were of grade 1-2 severity and were managed using standard practice or by withholding a dose of study treatment.
Limitations: This analysis is post hoc and exploratory in nature. Further, in this trial, PD-L1 expression did not provide predictive value for atezolizumab efficacy,5 so there remains a need for improvement of selection of previously platinum-treated patients who would benefit the most from atezolizumab monotherapy.
Interpretation for patient care: These long-term OS and safety findings were consistent with those from the primary analysis of IMvigor211.4 The greater potential for long-term survival seen with atezolizumab than chemotherapy in the ITT population and PD-L1 subgroups after > 2.5 years of follow-up support its recommended use in platinum-treated patients with mUC, regardless of PD-L1 status.5
Disclosure: Michiel S. van der Heijden has received institutional research funding from Astellas Pharma, AstraZeneca, Bristol Myers Squibb, and Roche; institutional fees for consulting or advisory roles from Astellas Pharma, AstraZeneca/MedImmune, Bristol Myers Squibb, Janssen, MSD Oncology, and Roche/Genentech; and travel, accommodation, and other expenses from Astellas Pharma, MSD Oncology, Novartis, and Roche.
The IMvigor211 study was funded by F Hoffmann-La Roche Ltd.
Editorial assistance was provided by Samantha Santangelo, PhD of Health Interactions, and was funded by F. Hoffman-La Roche, Ltd.
Written by: Michiel S. van der Heijden, Netherlands Cancer Institute, Amsterdam, The Netherlands
References:
- Witjes, J.A., et al. EAU guidelines on muscle-invasive and metastatic bladder cancer. European Association of Urology. 2021 May 2021]; Available from: https://uroweb.org/guideline/bladder-cancer-muscle-invasive-and-metastatic/.
- ESMO clinical practice guidelines. Genitourinary Cancers eUpdate – Bladder Cancer Treatment Recommendations. 2019 May 2021]; Available from: https://www.esmo.org/guidelines/genitourinary-cancers/bladder-cancer/eupdate-bladder-cancer-treatment-recommendations.
- National Comprehensive Care Network. NCCN Clinical Practice Guidelines in Oncology. Bladder Cancer. V2.2021. . 2021 May 2021]; Available from: https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf.
- Powles, T., et al., Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet, 2018. 391(10122): p. 748-757.
- van der Heijden, M.S., et al., Atezolizumab Versus Chemotherapy in Patients with Platinum-treated Locally Advanced or Metastatic Urothelial Carcinoma: A Long-term Overall Survival and Safety Update from the Phase 3 IMvigor211 Clinical Trial. Eur Urol, 2021.
Read the Abstract


