As prognostic and predictive factors, several tissue-based biomarkers have been studied in urothelial carcinoma (UC) for better patient selection. Although tissue testing has several advantages, it also presents challenges and limitations.
These include inter and intra-tumoral heterogeneity, dynamic changes during treatment, risks of complications related to the procedure, and limited or low-quality material availability. Liquid biopsy is emerging as a promising alternative in this context, especially the use of ctDNA in multiple cancer types (Figure 1).
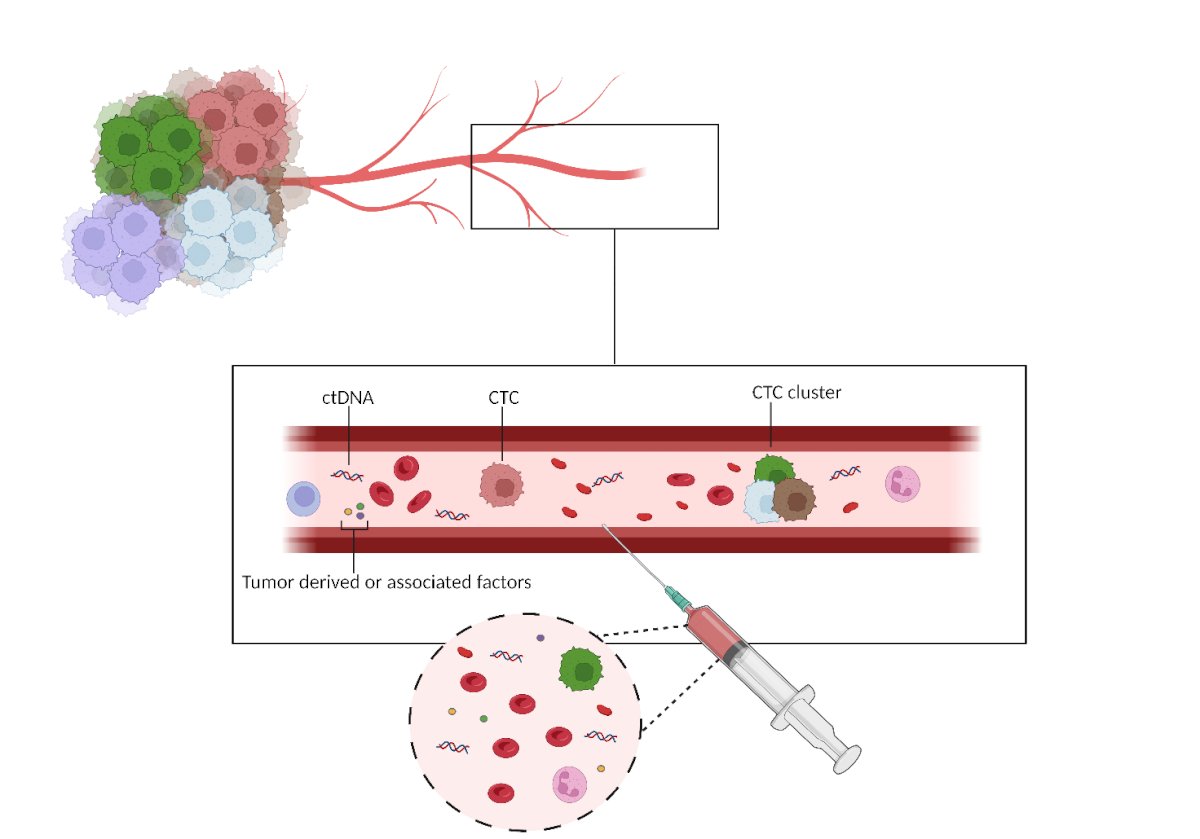
Figure 1- Liquid biopsy proof of concept: In this figure, an exemplified proof of concept scheme of how liquid biopsy is performed
CtDNA= circulating tumor DNA; CTC =Circulating Tumor Cell
While perioperative strategies for muscle-invasive bladder cancer (MIBC) have shown benefits, they can significantly impact patients' quality of life due to their toxicities. Identifying biomarkers to predict and monitor this condition other than to better select patients would be highly beneficial. This will enable the implementation of de-escalation strategies and prevent patients from experiencing any unnecessary toxicity.
In our Systematic Review, we assess the evidence on the prognostic and predictive role of ctDNA in the perioperative setting of MIBC after neoadjuvant and/or adjuvant chemotherapy and/or immunotherapy. [For more comprehensive information regarding the study protocol, please refer to the PROSPERO Register and the full text of the paper; Registration ID CRD42023391817].
As detailed in the publication, our review confirms the prognostic role of ctDNA in the post-cystectomy time-point. It shows a potential predictive benefit in the neoadjuvant chemotherapy and preoperative immunotherapy setting. ctDNA status and ctDNA dynamic showed encouraging evidence as a monitoring tool for recurrence, and to detect minimal residual disease, anticipating radiological progression with a significant median time difference in various trials.
IMvigor010 (NCT02450331) is a randomized multicenter, open-label, phase 3 trial comparing adjuvant atezolizumab to observation after surgical resection for operable urothelial cancer. Notably, a subgroup analysis of IMvigor010 trial showed that only ctDNA-positive patients treated with atezolizumab had an improvement in DFS (HR = 3.36 95% CI: 2.44–4.62). Moreover, clearance of ctDNA after two cycles of adjuvant atezolizumab was associated with improved outcomes in terms of DFS (HR = 0.26 95% CI: 0.12−0.56, p = 0.0014) and OS (HR = 0.14, 95% CI: 0.03–0.59).These findings underline the potential of ctDNA as a valuable tool in guiding treatment strategies in MIBC. By identifying ctDNA-positive patients, clinicians could better tailor treatment approaches to those most likely to benefit. This personalized approach, guided by ctDNA analysis, can potentially improve treatment outcomes and spare patients from unnecessary toxicities associated with treatments that may not be effective for them.
As precision medicine advances, the therapeutic landscape is expected to undergo significant changes in the next few years. Further prospective large studies are warranted, and the use of ctDNA has the potential to impact future research in this field significantly.
Written by: Emanuele Crupi, MD, Department of Medical Oncology, IRCCS San Raffaele University Hospital, Milan, Italy
Read the Abstract


