The absence of residual tumor in the surgical resection specimen, i.e. pathological complete response (pCR), after chemotherapy, is associated with improved outcome: patients with UC of the bladder who show a pCR had a 5-year overall survival rate of 70-80%,1 whereas patients with residual disease (≥ ypT2) or nodal metastases (>ypN0) had a 5-year overall survival rate of 15-20%.2 Currently, only 15% of patients with locally advanced or clinically node-positive UC achieve a pCR.1 This clearly demonstrates the unmet need to increase the proportion of patients achieving a pCR, thereby improving their clinical outcomes.
The Javelin 100 Bladder trial included patients with locally advanced irresectable or metastatic muscle-invasive bladder cancer (MIBC) who had at least stable disease after platinum-based chemotherapy and thereafter were treated with avelumab maintenance therapy.3 Sequential chemo-immunotherapy treatment in this population resulted in prolonged median overall- and progression free survival compared to patients who received the best supportive care following chemotherapy. The Chemotherapy And Sequential ImmunoTherapy for locally advanced urothelial cancer (CHASIT) study aims to translate these beneficial outcomes to patients with locally advanced or node-positive UC who are eligible to undergo radical surgery.
The CHASIT study is a prospective, multicenter, non-randomized single arm phase II clinical trial with the aim to improve the pCR rate in patients with locally advanced irresectable or clinically node-positive urothelial cancer by sequential treatment with chemo-immunotherapy. Patients with stage cT4NxM0 or cTxN1-N3M0 urothelial carcinoma of the bladder, urethra, or upper urinary tract, who have at least stable disease after treatment with a minimum of 3 and a maximum of 4 cycles of platinum-based chemotherapy, are eligible for inclusion. Following chemotherapy, patients receive 3 cycles of avelumab, which in the absence of disease progression is followed by surgical resection of the primary tumor and the affected lymph nodes. Follow-up consists of imaging every 3 months for 2 years. The primary endpoint is the pCR rate at radical surgery. Secondary endpoints include progression-free, cancer-specific, and overall survival at 24 months and the safety and tolerability of avelumab. It is hypothesized that sequential chemo-immunotherapy results in a pCR rate of ≥ 30%. A one sample proportion test will be used to compare the historical rate of 15% to the study arm. To obtain a power of 80%, 64 patients are screened and 58 patients are included in the efficacy analysis. An interim analysis is planned after the inclusion of the first 40 patients (75% of total accrual). If the pCR rate is <15% at interim analysis, the study will terminate prematurely. The study opened for inclusion in December 2022. The estimated date of last inclusion is December 2024.
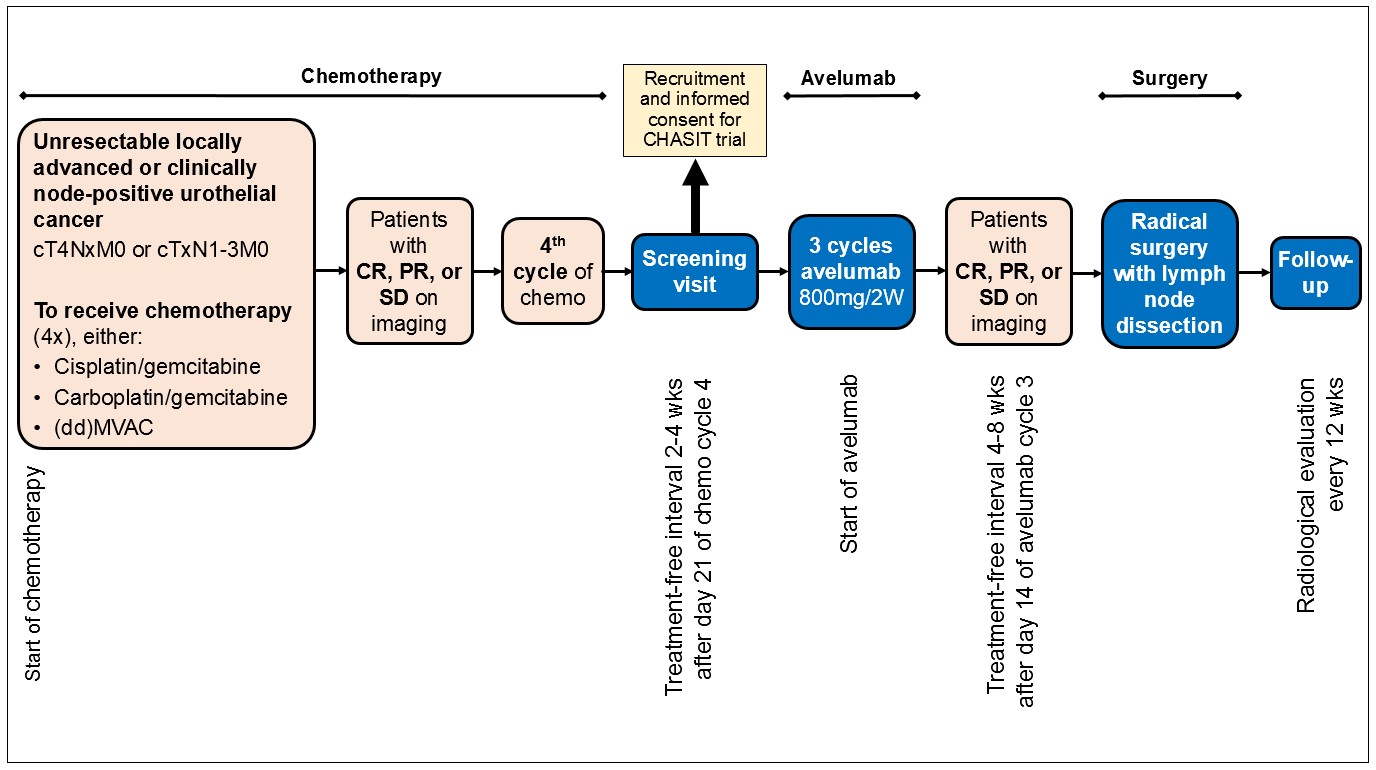
The CHASIT is the first study to assess the efficacy of sequential chemo-immunotherapy in the induction setting of UC. If the primary endpoint of the study is met, i.e. a pCR rate of ≥ 30%, a randomized controlled trial is foreseen in which this new treatment regimen is compared to the current standard of care.
Written by: V.C. Rutten, T.C.M. Zuiverloon, & J.L. Boormans
Department of Urology, Erasmus MC Cancer Institute, Rotterdam, the Netherlands
References:
- Zargar-Shoshtari K, Zargar H, Lotan Y, Shah JB, van Rhijn BW, Daneshmand S, et al. A multi-institutional analysis of outcomes of patients with clinically node positive urothelial bladder cancer treated with induction chemotherapy and radical cystectomy. J Urol. 2016;195:53–9.
- Meijer RP, Mertens LS, van Rhijn BW, Bex A, van der Poel HG, Meinhardt W, et al. Induction chemotherapy followed by surgery in node positive bladder cancer. Urology. 2014;83(1):134–9.
- Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab maintenance therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. 2020;383(13):1218–30.


