During the recruitment phase of the BladderGATE clinical trial (NCT 04134000), which investigated the efficacy of atezolizumab combined with BCG in patients with high-risk NMIBC, we collected various samples including urine, blood, tumor, and non-pathological tissues during surgery. As we needed to identify patients with high-risk NMIBC before receiving the final pathological report, we devised a method using cytology to stratify risk before surgery.
We prospectively gathered data from all patients scheduled for transurethral resection of the bladder (TURB) at our institution over two years. Subsequently, we calculated sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic odds ratio (DOR). We generated ROC curves for predicting high-risk using positive and suspicious cytology, as well as predicting low-risk using negative and atypical cytology. Additionally, we performed a combined analysis of positive and suspicious cytology to enhance the prediction of high risk.
Our study included a total of 224 patients. We found that the positive voided urine cytology (VUC) subgroup demonstrated a specificity of 92.4% (95% CI: 83.2-97.5%) and a PPV of 91.4% (95% CI: 81.0-97.1%), with a DOR of 6.81. In the suspicious VUC subgroup, specificity was 90.9% (95% CI: 81.3-96.6%), PPV was 88% (95% CI: 75.7-95.5%), and DOR was 4.23. Combining positive and suspicious cytology yielded a sensitivity of 65% (95% CI: 57.3-73.2%) and a DOR of 9.51 for detecting high-risk non-muscle-invasive bladder cancer. Negative VUC demonstrated high specificity in detecting low-risk cases (93.2%, 95% CI: 87.9-96.7%) with a DOR of 6.90 (95% CI: 3.07-15.46). However, atypical VUC exhibited lower specificity and predictive values, rendering it the least accurate.
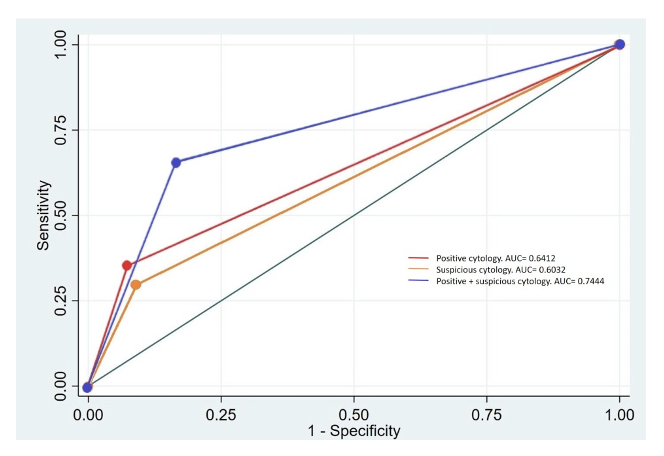
These findings have potential clinical implications. Patients suspected of having high-risk bladder cancer could be prioritized for surgery based on cytology results, or, as in our case, if during a scientific evaluation, patients have to be identified as high-risk before surgery, it can be a useful tool. Conversely, patients identified as low risk may require a single instillation of Mitomycin C, as it may only benefit low and intermediate-risk patients. We propose that integrating cytology as a risk prediction tool can aid clinicians in decision-making and advocate for cytology assessment if not already conducted during diagnosis.
Written by: Carmen Gómez del Cañizo, Professor, Urology Department, University Hospital 12 de Octubre, Madrid, Spain
Read the Abstract


