Healthcare – with its need for advanced resources and efficiency – is seemingly a perfect home for AI. But the sector has been slow on digitalizing, let alone bringing in such game-changing technologies. Yet gradually, as healthcare facilities around the world digitalize their processes, we’re seeing not just a new way of getting results, but a new way of thinking.
This is now being demonstrated in bladder cancer care with advanced tools emerging that soon expect to become the new norm. In uro-oncology, AI can have a variety of utilities, such as aiding pre-surgical planning through 3D-imaging, and improving the quality of procedures through augmented reality guidance.1,2 In addition, utilizing AI in diagnostics can provide support through real-time tumor detection, histological categorization, risk stratification, and treatment planning.3,4
So, how can AI and diagnostic technologies help when it comes to the care pathway in non-muscle-invasive bladder cancer (NMIBC)? To see the potential in this field, it’s important to first understand the context of the disease itself.
Why is it crucial to address challenges in NMIBC?
NMIBC remains a major health concern worldwide. In fact, recurrences and progression to muscle invasive bladder cancer (MIBC) affect nearly half of the diagnosed NMIBC population, potentially due to misdiagnosis, delay of diagnosis and incomplete resection of tumors.5–7 In NMIBC, missing early recurrence of high-risk lesions may lead to substantial delay of appropriate treatments, which may have a detrimental impact on disease prognosis.8–10
NMIBC reportedly has the highest lifetime cancer treatment costs per patient due to the frequency of procedures and interventions11,12
A delay in bladder cancer diagnosis is more common among women and patients in rural or resource-poor areas13,14
So what can be done to address this? Accurate, timely diagnosis and prediction of recurrence and progression is essential in NMIBC management,12 and advanced tools that can help us to achieve this are key4 – this is where the use of AI is beginning to show promise.4,12
The need for early, accurate diagnosis and improved risk stratification
Expert consensus and NMIBC guidelines are aligned on there being a need for improving the overall quality of NMIBC care and diagnostics in particular.15,16
Identifying patients’ risk factors, or ‘risk stratification’, is vital when planning, predicting prognosis and outcomes, and managing treatment for NMIBC.17 However, current models to support risk stratification have limitations. There’s no universally accepted standard, and the significance of various risk factors used in such models isn’t always clear.18,19 Current models also still largely exclude molecular and genomic profiling of bladder tumors17,20 – factors which can be crucial for identifying a targeted treatment approach. This can lead to delayed or suboptimal treatment – resulting in worse outcomes for NMIBC patients and higher costs of care.18
Early and precise diagnosis of NMIBC is essential for accurate risk stratification, decision making and treatment planning. This in turn may enhance the effectiveness of treatment, by identifying patients who are unlikely to benefit from specific treatments and avoiding unnecessary procedures – leading to more cost-effective care.21,22
Shifting to a precision-based approach in NMIBC
Improving diagnostic precision is becoming increasingly important in NMIBC. NMIBC care is just starting to tap into modern technologies like advanced genetic testing and is expected to further shift with the emergence of new targeted treatment options and technological advancements like AI.23
What is precision diagnostics in NMIBC?
Precision diagnostics doesn’t just consider the clinical presentation and traditional risk factors, it allows a more thorough understanding of the genetic and molecular characteristics of tumors and identifies abnormalities that might be causing it to be more aggressive.24
How? By utilizing current conventional diagnostics such as radiology and cystoscopy, in combination with emerging tools including next-generation imaging techniques, biomarkers based on immunohistochemistry, next-generation sequencing (NGS), multi-omics, and leveraging artificial intelligence (AI)/machine learning (ML).24
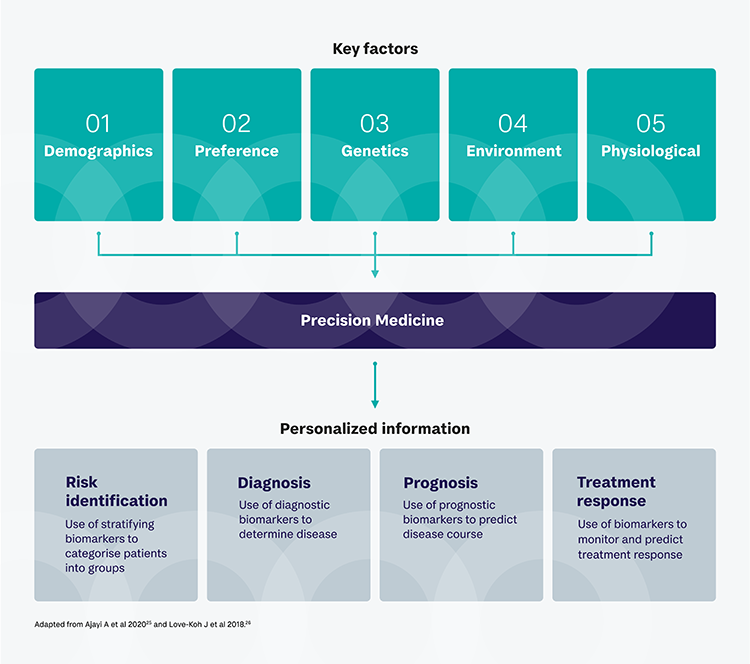
A precision-based approach aims to get greater effectiveness from treatment and less off-target effects. The rapid advancement of technologies, the shift towards precision medicine and emerging novel targeted treatments in NMIBC all drive the renewed emphasis of the importance of the diagnostic process.
“The ability to improve the management of NMIBC is upon us with all the new technologies and medicines entering the market. Novel diagnostic tools, including AI, offer clinicians increasing amounts of information for risk assessment and decision making. Optimizing the diagnosis to identify patients who could benefit from novel therapies will have a positive impact on the prognosis for bladder cancer patients.” - Dan Schneider, President and CEO of PhotocureWhat are the benefits?
Precision diagnosis, along with increasing support from AI, may bring us closer to addressing the challenges faced in NMIBC care. It can identify patients who are more likely to respond, or unlikely to benefit, to particular treatment. Plus, more individualized and targeted therapies can increase the chance of a response to treatment while reducing side effects as opposed to more general approaches like chemotherapy.24
A shift towards precision diagnostics and personalized treatment is clearly already underway, with approvals of targeted drugs such as erdafitinib and pembrolizumab in NMIBC,27 as well as AI-based diagnostic tools being explored in several areas of uro-oncology, including prostate and bladder cancer.23,28
Transforming NMIBC care with AI
Advancement in technology, with the use AI and ML, has the potential to dramatically shape medical procedures in the near future. AI and ML have the potential to revolutionize NMIBC management and are increasingly being used to analyze images and other data, and can help identify tumors more accurately, highlighting high-risk patients, enhancing clinical decision making, and fast-tracking patients to more effective targeted treatments.3,4
AI-supported software can enhance current surgical procedures. It can integrate findings from imaging, such as blue light cystoscopy (BLC), with other patient data, such as medical history and pathology grading, to identify patients at risk and predict outcomes.58 There are also increasing advancements in AI-based tools to support clinical decision making, with one tool already available and recommended by NCCN guidelines for prostate cancer. This tool, ArteraAI Prostate Test, analyzes digital pathology images to help identify patients who will benefit from therapy and guide treatment decisions.23
In NMIBC specifically, AI is being increasingly explored in clinical trials, with one tool evaluated in a recent clinical study that uses more comprehensive molecular profiling to evaluate pre- and post-treatment responses to BCG immunotherapy. It aims to create a personalized model to show presence of NMIBC and predict response to BCG.28 Another tool, PROGRxN-BCa (PROGression Risk assessment in NMIBC), presented at the 2024 AUA congress exceeded current tools in NMIBC when predicting disease progression, demonstrating a benefit in avoiding unnecessary treatment escalation.59 In addition, a recent systematic review of AI studies in NMIBC found AI models to generally outperform non-AI models overall when predicting NMIBC outcomes.12
“AI is intended to complement clinical practice, not replace decision making by clinicians. By combining AI with enhanced imaging technology and biomarkers, it can help achieve more precise diagnoses, help analyze and integrate the increasing amount of data, support decision making and improve overall outcomes for patients in the long term.” - Anders Neijber, Chief Medical Officer of PhotocureAs we look to the future, there are still hurdles to overcome when it comes to broader implementation of these technologies and shifting to precision diagnostics. This includes limited-quality clinical data, lack of guideline recommendations, high equipment costs and lack of regulatory approvals.60
Despite rapid increases in evidence around the use of these tools, there is still room for improvement, and there is a clear need for collaboration between healthcare and AI communities to develop higher quality models, allowing us to reach the next step in NMIBC care.12
Conclusion
With NMIBC remaining a significant health concern globally, the integration of novel diagnostic technologies and AI promises to revolutionize diagnosis and treatment. By enhancing accuracy, integration of data, aiding risk stratification, and guiding personalized treatment approaches, these advancements offer hope for improved patient outcomes and more efficient disease management overall. While challenges such as data quality, cost, utility and regulatory hurdles exist, the trajectory is clear: the combination of AI and precision diagnostics procedures holds immense promise for transforming the landscape of NMIBC care in the years to come.
References
- Porpiglia F et al. Eur Urol Focus. 2018;4(5):652–656.
- Chahal B et al. Curr Opin Urol. 2024;34(1):32–36.
- Wu S et al. J Natl Cancer Inst. 2022;114(2):220–227.
- Laurie MA et al. Urol Clin North Am. 2024;51(1):63–75.
- Shalata AT et al. Cancers (Basel). 2022;14(20):5019.
- Cumberbatch MGK et al. Eur Urol. 2018;73(6):925–933.
- Sylvester RJ et al. Eur Urol. 2006;49(3):466–477.
- Williams SB et al. JAMA Netw Open. 2021;4(3):e213800.
- van den Bosch S et al. Eur Urol. 2011;60(3):493–500.
- Morelli M et al. J Endourol. 2021;35(12):1824–1828.
- Sievert KD et al. World J Urol. 2009;27(3):295–300.
- Kwong JCC. NPJ Digit Med. 2024;7:98.
- World Bladder Cancer Patient Coalition. Patient & carer experiences with bladder cancer: findings from a global survey. 2023. Available at: https://worldbladdercancer.org/wp-content/uploads/2023/06/WBCPC-Patient-Survey-Report-Executive-Summary-2.pdf. Accessed May 2024.
- Deuker M et al. Cancer Causes Control. 2021;32(2):139–145.
- National Institute for Health and Care Excellence (NICE). Bladder cancer: diagnosis and management [NG2]. Published: February 2015. Available at: https://www.nice.org.uk/guidance/ng2/chapter/1-recommendations. Accessed May 2024.
- National Institute for Health and Care Excellence (NICE). Improving outcomes in urological cancers. Available at: https://www.nice.org.uk/guidance/csg2/resources/improving-outcomes-in-urological-cancers-pdf-773372413. Accessed May 2024.
- Sylvester RJ et al. Eur Urol. 2021;79(4):480–488.
- Russell B et al. Eur Urol Oncol. 2020;3(2):239–249.
- Isharwal S, Konety B. Indian J Urol. 2015;31(4):289–296.
- Soukup V et al. Eur Urol Focus. 2020;6(3):479–489.
- Gontero P et al. EAU guidelines on non-muscle-invasive bladder cancer (TAT1 and CIS). April 2024.
- Holzbeierlein J et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline: 2024 amendment. J Urol. 2024.
- ArteraAI. Available at: https://artera.ai/. Accessed May 2024.
- Geneseeq. Precision oncology. Available at: https://na.geneseeq.com/precision-oncology/. Accessed May 2024.
- Ajayi A et al. Precision medicine of autoimmune diseases. 2020. 10.5772/intechopen.95248.
- Love-Koh J et al. Pharmacoeconomics. 2018;36(12):1439–1451.
- Zhao P et al. J Hematol Oncol. 2019;12(1):54.
- BioSpace. Ludwig enterprises mRNA bladder cancer study to be launched. 2024. Available at: https://www.biospace.com/article/releases/ludwig-enterprises-mrna-bladder-cancer-study-to-be-launched/. Accessed May 2024.
- Gofrit ON et al. J Urol. 2010;183(5):1678–1684.
- Roumiguié M et al. Eur Urol 2022;82(1):34–46.
- Llano A et al. Cancers (Basel). 2024;16(2):245.
- PR Newswire. Bladder cancer: Long-term benefits of blue light cystoscopy and enhanced detection with HD technology unveiled at AUA 2024. Available at: https://www.prnewswire.com/news-releases/bladder-cancer-long-term-benefits-of-blue-light-cystoscopy-and-enhanced-detection-with-hd-technology-unveiled-at-aua-2024-302137774.html. Accessed May 2024.
- Hu X et al. Cancers (Basel). 2022;14(13)3181.
- Laser Focus World. Image analysis software offers multiplex cell detection. 2024. Available at: https://www.laserfocusworld.com/bio-life-sciences/article/14304543/leica-microsystems-gmbh-image-analysis-software-offers-multiplex-cell-detection. Accessed May 2024.
- Mulawkar PM et al. Front Surg. 2022;9:762027.
- Medtronic. GI GeniusTM intelligent endoscopy module. Available at: https://www.medtronic.com/covidien/en-gb/products/gastrointestinal-artificial-intelligence/gi-genius-intelligent-endoscopy.html#. Accessed May 2024.
- Chang S et al. Sci Rep. 2023;13:21484.
- Nalepa J. Sensors. 2021;21(18):6002.
- Ahmadi H, Daneshmand S. Transl Androl Urol. 2021;10(1):1–6.
- Dexter A et al. J Mol Pathol. 2022;3(3):168–181.
- González-Cerdas GE et al. J Optic Micro. 2023;011006.
- Droller MJ. Urol Oncol. 2021;39(9):506–513.
- Schmidbauer J et al. Eur Urol. 2009;56(6):914–919.
- Schubert T et al. Ther Adv Urol. 2017;9(11):251–260.
- Hosny A et al. Nat Rev Cancer. 2018;18(8):500–510.
- Wong VK et al. Cancers (Basel). 2021;13(6):1396.
- Shahait M et al. J Egypt Natl Canc Inst. 2023;35(1):21.
- Kalokairinou K et al. Adv Urol. 2014;923958.
- Tufano A et al. Curr Oncol. 2024;31(2):818–827.
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource [Internet]. Silver Spring (MD): Food and Drug Administration (US); 2016-. Glossary. 2016 Jan 28 [Updated 2021 Nov 29].
- Ciocan-Cartita CA et al. Int J Mol Sci. 2019;20(10):2576.
- Clinicaltrials.gov. NCT02203136. Available at: https://www.clinicaltrials.gov/study/NCT02203136. Accessed May 2024.
- LabMedica. Breakthrough in diagnostic technology could make on-the-spot testing widely accessible. Available at: https://www.labmedica.com/technology/articles/294799462/breakthrough-in-diagnostic-technology-could-make-on-the-spot-testing-widely-accessible.html. Accessed May 2024.
- Seamaty. The future of healthcare: point-of-care diagnostics and devices. 2023. Available at: https://en.seamaty.com/index.php?s=/sys/543.html. Accessed May 2024.
- Healthcare in Europe. Emerging technologies in POCT. Available at: https://healthcare-in-europe.com/en/news/emerging-technologies-in-poct.html. Accessed May 2024.
- Wahab MRA et al. Pathol Res Pract. 2023;250:154812.
- Jonas O et al. Sci Transl Med. 2015;7(284):284ra57.
- Takeuchi M, Kitagawa Y. Ann Gastroenterol Surg. 2024;8:4–5.
- Kwong JCC et al. Abstract: PD30-05. Presented at AUA congress, May 2024.
- Ferro M et al. Diagnostics (Basel). 2023;13(13):2308.


