LELC-B is a rare histologic subtype characterized by strong immune cell infiltrates and syncytial growth of the tumor cells (Figure 1).
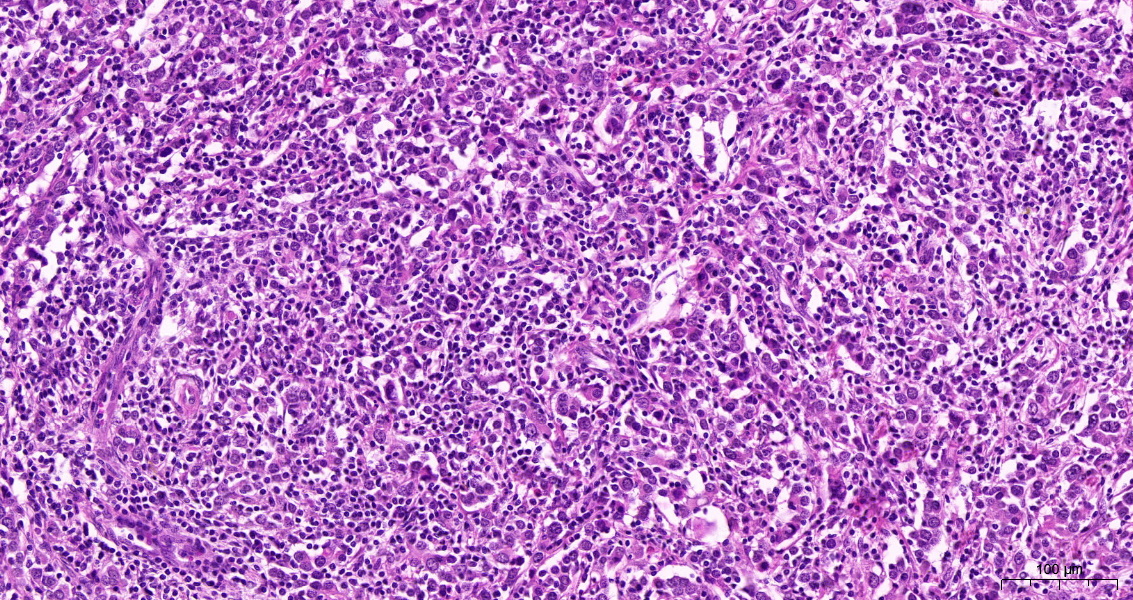
It usually shows a better prognosis and favorable response rates to immune-checkpoint inhibitors (ICI) compared to the usual urothelial carcinoma without subtypes (not-otherwise-specified, NOS). Similar tumors in other organs, such as lymphoepithelial carcinomas of the head and neck exist, however, seemingly with a different molecular background and associations with viral infections (such as EBV).
In the present study, we wanted to comprehensively characterize the molecular profiles and immune cell infiltration of LELC-B to achieve a better understanding of the tumor and its therapeutic implications. Therefore, we analyzed 11 muscle-invasive bladder cancer cases with LELC-B. We used different technologies such as immunohistochemistry to evaluate the PD-L1 expression and status of the mismatch-repair (MMR) proteins. The EBV status was analyzed by in-situ hybridization (EBER). In addition, we performed whole exome sequencing of the LELC-B cases and for comparison also of 10 NOS cases. Transcriptomic signatures were analyzed with NanoString nCounter PanCancer IO360 panel. In addition, the tumor microenvironment (TME) was evaluated by multiplex immunofluorescence regarding the TME itself (PD-L1, Ki67, aSMA, PanCK, CD45, Vimentin) and focused on T-cells (CD8, CD4, CD3, CD163, PD-1, FoxP3) to quantify cell populations.
We found that all LELC-B cases strongly expressed PD-L1 (median TPS/TC 70%; range 20-100; median CPS 100; range 50-100), were all MMR-proficient, and negative for EBV.
These results are important, as a high PD-L1 expression usually is an indicator of a more likely response to ICI. That lack of MMR deficiency shows that microsatellite-instability (MSI) does not seem to be a hallmark of LELC-B. These findings have in part been shown in previous studies of LELC-B. The lack of EBV-infection in LELC-B has also been described and is now underlined by our data, showing a clear difference from similar tumors in other sites with regular association with viral infection.
In multiplex immunofluorescence analyses, we found a high CD8+ T-cell count and high PD-1/PD-L1 expression on immune and tumor cells. LELC-B exhibited upregulation of signaling pathways that are important in immune cell response in transcriptomic analyses. The mutational profile was similar to that of NOS urothelial cases with most common mutations in chromatin remodeling genes that are known to cause epigenetic dysregulation.
Most importantly, all LELC-B cases showed a high tumor mutational burden of 39 Mut/Mb (IQR 29-66), which was higher than in usual urothelial carcinoma NOS. Although we did not detect the reason for the high TMB in LELC-B, it might be used as a predictive biomarker indicating the potential usefulness of ICI.
In conclusion, we showed that LELC-B is a highly immunogenic tumor, that has a strong upregulation of PD1/PD-L1 and high TMB with the potential usefulness of ICI.
It is the largest study of its kind to date addressing specifically LELC-B.
Written: Prof. Henning Reis, MD, University Hospital Frankfurt, Dr. Senckenberg Institute of Pathology, Frankfurt, Germany
Read the Abstract


