Radical cystectomy, the current standard for MIBC, carries a 50% systemic relapse rate within three years. Neoadjuvant chemotherapy has historically improved survival outcomes but remains underutilized. NIAGARA expands on this foundation by incorporating immunotherapy, leveraging its potential to eradicate micrometastatic disease and enhance antitumor immune responses preoperatively.
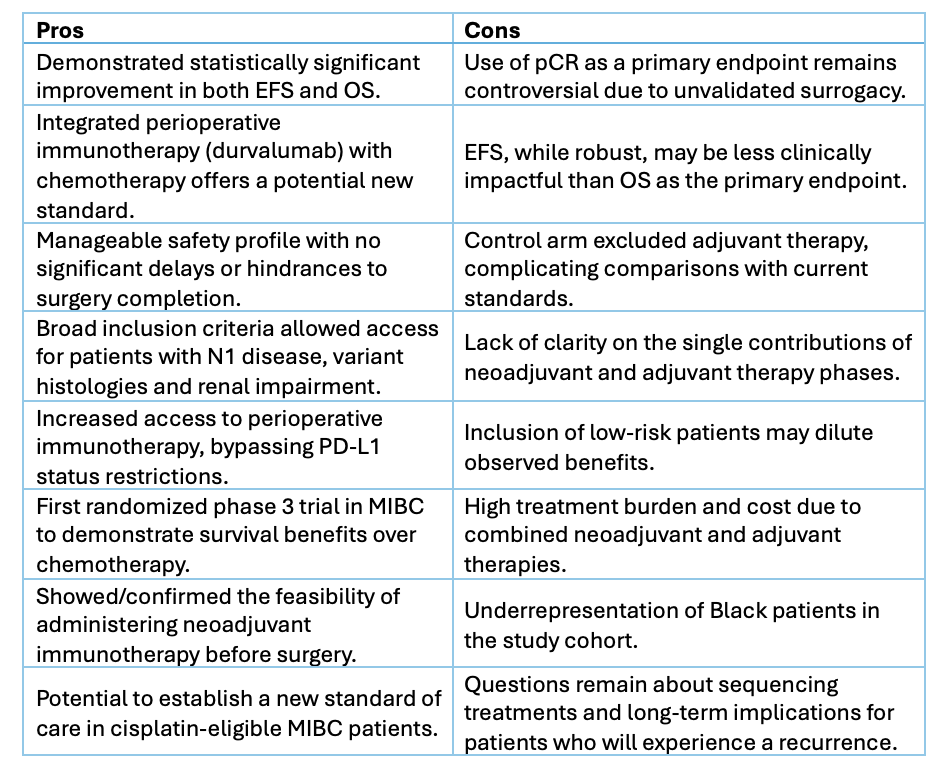
NIAGARA randomized 1,063 patients to receive either perioperative durvalumab plus chemotherapy or chemotherapy alone, followed by surgery. While pathologic complete response (pCR) rates were secondary endpoints, the trial primarily focused on EFS—a more clinically meaningful measure of treatment success. Notably, the inclusion of durvalumab improved 24-month EFS (67.8% vs. 59.8%) and OS (82.2% vs. 75.2%), with a manageable safety profile and no delays in surgery.
Despite its strengths, the study highlights critical questions. The appropriateness of pCR as an endpoint in phase 3 trials is debated due to its unvalidated surrogacy for survival outcomes. Furthermore, while EFS is robust, OS remains the gold standard for guiding clinical practice. The trial's design also leaves unanswered the individual contributions of neoadjuvant and adjuvant therapies, raising concerns about treatment burden and cost.
The study broadens access to perioperative immunotherapy, circumventing barriers like PD-L1 status restrictions. However, it may include patients with low-risk profiles, potentially diluting its impact. Additionally, the trial’s control arm excluded adjuvant therapy, a current standard for high-risk MIBC, complicating direct comparisons to contemporary practices.
Future directions should prioritize trials incorporating biomarkers, such as circulating tumor DNA (ctDNA), to refine patient selection and optimize therapy. Ongoing studies like KEYNOTE-B15 may redefine paradigms by integrating antibody-drug conjugates and ICIs while bladder-sparing approaches promise enhanced quality of life for selected patients.
In conclusion, NIAGARA represents a significant step forward, demonstrating a clear survival benefit and potentially setting a new benchmark in MIBC care. Whether this trial marks a transformative milestone or a transitional phase in oncologic practice remains a question only further research will answer.
Written by:
- Daniele Raggi, MD, Department of Genitourinary Medical Oncology, The Royal Marsden Hospital, London, United Kingdom
- Prof. Robert A. Huddart, PhD, Professor, Department of Genitourinary Medical Oncology, The Royal Marsden Hospital, The Institute of Cancer Research, London, United Kingdom


