BERKELEY, CA (UroToday.com) - The efficacy of bacillus Calmette-Guerin (BCG) in the treatment of high-grade, non-muscle invasive bladder cancer (NMIBC) is unquestioned by the urologic community today, 35 years after its implementation. However, many questions still remain and the optimal BCG regimen is still under constant debate. Approximately 5-10% of patients will sustain an adverse side effect and approximately 30-50% will progress to muscle invasion. Worldwide, only 40% of patients undergo maintenance therapy, with the majority occurring in Europe according to recommendations of the European Association of Urology (EAU).[1] Despite the effectiveness proven of BCG, many questions remain regarding the optimal dosing schedule and regimen that will limit toxicity while maintaining the therapeutic effect.
Local toxicity is common with BCG use and most often self-limiting. These irritative symptoms can become extremely debilitating or progress to systemic toxicity, therefore precluding further treatment. The CUETO randomized, prospective trial found an 18.4% difference between local severe toxicity experienced favoring patients receiving 1/3 dose BCG. In assessing 500 patients with a median follow-up of 18.6 months, they found no significant differences in recurrence rate (18.1% BCG full dose versus 19.5% BCG 1/3 dose) or progression rate (2.4% versus 4.8%).[2] Martinez-Pinero et al.confirmed their previous findings by recently demonstrating that 1/3-dose BCG is as effective as full-dose in the prevention of disease recurrence (45% and 39%, respectively) and progression (26% and 24.7%, respectively),[3] findings replicated by multiple other studies.[4, 5, 6] Increasing instillation intervals[7] and decreasing bladder dwell times[8] have also been means to reduce the rate of local toxicity. Recently, Lamm et al. demonstrated preliminary findings regarding the utilization of heat-inactivated BCG in 29 patients once deemed BCG intolerant.[9] The study found 100% patient tolerance, and in comparison to patients receiving live-BCG, a reduction in grade 2 local and systemic side effects along with the elimination of grade 3 systemic side effects. No significant difference was noted in tumor recurrence, progression, or overall survival. With demonstration of the effectiveness of the various modalities of BCG administration, it appears that toxicity can sufficiently be reduced in a manner that preserves therapeutic efficacy, even in patient for whom BCG appears to be contraindicated.
According to the National Cancer Comprehensive Network,[10] maintenance BCG therapy is optional for complete responders. All randomized studies utilizing schedules other than the 3 weekly instillations outlined in the SWOG 8507 trial,[11] have failed to demonstrate that maintenance reduces disease recurrence, and no trial has replicated the effect on progression without using 3-week maintenance. Failure to understand this difference has complicated and confounded the establishment of standard practices within the urologic community. Meta-analyses of studies, including the 3-week maintenance schedule, however, have consistently demonstrated the effect of tumor recurrence reduction.[12, 13, 14] Additionally, multiple recent randomized controlled trials investigating maintenance therapy according to the SWOG protocol have reproduced the long-term significant reduction in recurrence.[15, 16, 17, 18] Interestingly, Di Stasi et al. have shown an effect of sequential maintenance BCG with electromotive mitomycin on disease-free interval (p < 0.01), recurrence (p < 0.01), progression (p < 0.01), overall mortality (p < 0.05), and cancer-specific mortality (p=0.01) with a median follow-up of 7 years.[19]
Recently, Herr et al. reported 5-year recurrence-free and progression-free survival data on 816 complete BCG responders (defined as negative biopsy and cytology at 6 months post induction BCG) who did not receive maintenance therapy.[20] By emphasizing newer advances in bladder cancer treatment including repeat resection, postoperative intravesical chemotherapy, expert wide tumor resection, and improved visualization with blue-light and narrow-beam imaging, the study questions the added value 3-week maintenance BCG has to offer. However, as it stands today, the 3-decade-old multicenter SWOG 8507 protocol remains the standard of care practice and has demonstrated success not replicated by any other form of treatment practice, namely statistically significant reduction in recurrence, metastasis, and prolongation of cancer-specific survival and overall survival.[21]
The EORTC-GU Cancers Group recently published the largest randomized trial bladder cancer data demonstrating a 64.2% 5-year disease free rate in patients receiving full-dose 3-year maintenance and 58.8% in those receiving full-dose 1-year maintenance.[22] Sub-group analyses found the benefits of full-dose BCG maintenance localized to 1 year in intermediate-risk patients (38% recurrence) and to 3 years in high-risk patients (34%) in terms of recurrence prevention (Figure 1), but no significant differences were noted in progression or survival. Interestingly, they found maintenance therapy to be extremely well tolerated (only 9.1% unable to continue full-dose therapy for 3 years), unlike in the SWOG study where only 16% enrolled to completion of 3 years, suggesting that with further experience, BCG has become better tolerated. With a median 9.2 year follow-up, a greater reduction in metastasis and mortality was noted for intermediate-risk patients when compared to higher-risk patients on maintenance,[15] however Oddens et al. recently showed no benefit to greater than 1-year maintenance in intermediate-risk patients over a median 7.1 year follow-up.[21] Taking these results into consideration, the EAU Guidelines now recommend 1-3 years full-dose maintenance for appropriate risk patients. An international physician practice-pattern survey demonstrated a significant non-adherence to EAU and AUA guideline recommendations regarding BCG use (only 47% intermediate-risk patients receiving BCG) and tolerability of those receiving maintenance therapy (15% discontinuation).[23] Needless to say, the debate continues as to the optimal duration and schedule of BCG treatment, and further randomized controlled trials are warranted to ascertain proper guidance and establishment of standardized protocols.
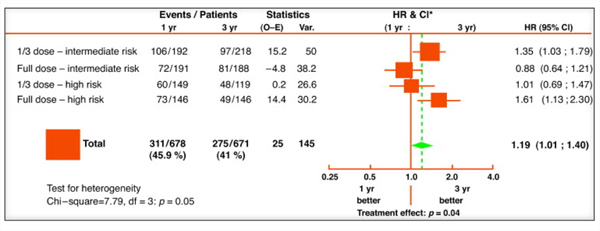 |
| Figure 1. Disease-free interval: 1 year of maintenance versus 3 year of maintenance according to dose and risk group.[22] (Click image to enlarge) |
References:
- Babjuk M et al. Guidelines on non-muscle-invasive bladder cancer. European Association of Urology 2013.
- Martinez-Pineiro JA et al. Improving the safety of BCG immunotherapy by dose reduction. Eur Urol 1995; 27 (suppl 1): 13-18.
- Martinez-Pineiro JA, Martinez-Pineiro L, Solsona E et al. Has a 3-fold decreased dose of Bacillus Calmette-Guerin the same efficacy against recurrences and progression of T1G3 and Tis bladder tumors than the standard dose? Results of a prospective randomized trial. J Urol 2005; 174: 1242-47.
- Martinez-Pineiro JA, Flores N, Isorna S et al. Long-term follow-up of a randomized prospective trial comparing a standard 81mg dose of intravesical Bacille Calmette-Guerin with a reduced dose of 27mg in superficial bladder cancer. BJU Int 2002; 89: 671-80.
- Takashi M, Wakai K, Ohno Y et al. Evaluation of a low-dose intravesical Bacillus Calmette-Guerin (Tokyo strain) therapy for superficial bladder cancer. Int Urol Nephrol 1995; 27: 723-33.
- Ojea A, Nogueira JL, Solsona E et al. A multicentre, randomized prospective trial comparing three intravesical adjuvant therapies for intermediate-risk superficial bladder cancer: low-dose Bacillus Calmette-Guerin (27mg) versus very low-dose Bacillus Calmette-Guerin (13.5mg) versus mitomycin C. Eur Urol 2007; 52: 1398-406.
- Bassi P. Spinadin R, Carando R et al. Modified induction course: A solution to side-effects? Eur Urol 2000; 37 (Suppl. 1): 31-2.
- Andius P, Fehrling M, and Holmang S. Intravesical Bacillus Calmette-Guerin therapy: Experience with a reduced dwell-time in patients with pronounced side-effects. BJUI Int 2005; 96: 1290-93.
- Lamm DL, Iverson T, and Wangler V. Clinical experience with heat-inactivated bacillus Calmette-Guerin (BCG) immunotherapy. AUA 2013 abstract.
- National Cancer Comprehensive Network Clinical Practice Guidelines in Oncology: Bladder Cancer. 2013.
- Lamm DL, Blumenstein BA, Crissman JD et al. Maintenance Bacillus Calmette-Guerin immunotherapy for recurrent Ta, T1 and carcinoma in situ transitional cell carcinoma of the bladder: A randomized Southwest Oncology Group Study. J Urol 2000; 163: 1124-29.
- Bohle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol 2003; 169: 90–5.
- Han RF, Pan JG. Can intravesical bacillus Calmette-Guerin reduce recurrence in patients with superficial bladder cancer? A meta-analysis of randomized trials. Urology 2006; 67: 1216–23.
- Malmstrom PU, Sylvester RJ, Crawford DE et al. An individual patient data meta-analysis of the long-term outcome of randomized studies comparing intravesical mitomycin C versus bacillus Calmette-Guerin for non-muscle-invasive bladder cancer. Eur Urol 2009; 56: 247–56.
- Sylvester RJ, Brausi MA, Kirkels WJ, et al. Long-term efficacy results of EORTC Genito-Urinary Group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guerin, and bacillus Calmette-Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol 2010; 57: 766-773.
- Hinotsu S, Akaza H, Naito S, et al. Maintenance therapy with bacillus Calmette-Guerin Connaught strain clearly prolongs recurrence-free survival following transurethral resection of bladder tumour for non-muscle-invasive bladder cancer. BJUI 2010; 108: 187-195.
- Koga H, Ozono S, Tsushima T, et al. Maintenance intravesical bacillus Calmette-Guerin instillation for Ta, T1 cancer and carcinoma in situ of the bladder: Randomized controlled trial by the BCG Tokyo Strain Study Group. Int J Urol 2010; 17: 759-67.
- Duchek M, Johansson R, Jahnson S, et al. Bacillus Calmette-Guerin is superior to a combination of epirubicin and interferon-α2b in the intravesical treatment of patients with stage T1 urinary bladder cancer. A prospective, randomized, Nordic study. Eur Urol 2010; 57: 25-31.
- Di Stasi SM et al. Sequential BCG and electromotive mitomycin versus BCG alone for high-risk superficial bladder cancer: a randomized controlled trial. Lancet Oncol 2006; 7 (1): 43-51.
- Herr HW, Dalbagni G, and Donat SM. Bacillus Calmette-Guerin without maintenance therapy for high-risk non-muscle-invasive bladder cancer. Eur Uro 2011; 60 (1): 32-36.
- Sylvester RJ et al. Long-term efficacy results of EORTC Genito-Urinary Group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guerin, and bacillus Calmette-Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol 2010; 57: 766-773.
- Oddens et al. Final results of an EORTC-GU Cancers Group randomized study of maintenance bacillus Calmette-Guerin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: One-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol 2013; 63 (3): 462-472.
- Witjes JA et al. Current clinical practice gaps in the treatment of intermediate- and high-risk non-muscle-invasive bladder cancer (NMIBC) with emphasis on the use of bacillus Calmette-Guerin (BCG): Results of an international individual patient data survey (IPDS). BJUI 2013. [Epub ahead of print].
Written by:
Nilay M. Gandhi, MD;a Alvaro Morales, MD;b and Donald L. Lamm, MDc as part of Beyond the Abstract on UroToday.com. This initiative offers a method of publishing for the professional urology community. Authors are given an opportunity to expand on the circumstances, limitations etc... of their research by referencing the published abstract.
aJames Buchanan Brady Urological Institute, Johns Hopkins Medical Institutions, Baltimore, MD, USA
bQueen's University, Kingston, ON, Canada
cBCG Oncology, University of Arizona, Phoenix, AZ, USA
Bacillus Calmette-Guérin immunotherapy for genitourinary cancer - Abstract

More Information about Beyond the Abstract


