It is estimated, that almost 25% of patients have micrometastases in inguinal lymph nodes at the time of diagnosis not possible to recognise during physical examination, therefore there is an urgent need to identify new morphological and molecular markers which would serve as early predictors of aggressive pSCC behavior. The administration of adjuvant treatment in high risk patients may be an example of the clinical use of these prognostic markers.
Like in head and neck SCC or vulvar SCC, in the penis may occur either HPV-associated SCC related with undifferentiated squamous intraepithelial lesion (SIL) and expression of oncoprotein p16 or HPV-negative SCC associated with chronic inflammation and differentiated penile intraepithelial neoplasia (d-PeIN). HPV-associated SCC constitutes about 30-80% of penile cancers in various studies.2,3 Positive p16 immunohistochemistry is a reliable and robust sign of HPV-associated SCCs.4 HPV-negative SCC arises probably based on multiple sporadic mutations predominantly in chronic inflammation. In penile carcinoma, the prognostic significance of tumor infiltrating lymphocytes (TILs) number is still somewhat unclear. Host immune system and especially the activity of cytotoxic T-cells is a pivotal factor influencing the prognosis and course of malignant disease. There are many articles documenting better prognosis in some other tumors with high numbers of TILs. This is in line with the assumption of active destruction of tumor cells by cytotoxic T-cells. The high number of TILs is a marker of highly active host immune response. This relationship was described in penile carcinoma.5 Moreover, there are more TILs in HPV-associated carcinomas tending to have a better prognosis.6 Epithelial-mesenchymal transition (EMT) has been described as a mechanism of metastasizing in various epithelial tumors. Moreover, EMT has been documented by several studies as a significant prognostic factor in epithelial tumors. Tumor budding is a histomorphological correlate of EMT, budding means the dropping of single tumor cells or small clusters into the surrounding stroma, occurring usually at the tumor invasion front. Tumor budding is well described by many studies as an unfavorable prognostic marker, i.e. in colorectal, esophageal, pancreatic, or uterine cervix cancer. The budding is probably the mechanism of how the tumor enters lymphatic or blood vessels at the beginning of metastatic spread.7 To our knowledge, there is no robust study assessing the prognostic impact of budding in penile carcinoma nowadays, probably given the low number of penile carcinomas. We hypothesized that in penile carcinoma, aggressive behavior and capability to metastasize depends on HPV status, extent of epithelial-mesenchymal transition (EMT), and host immune response.
We aimed to assess the prognostic significance of tumor budding, intratumoral and peritumoral immune cell density, immunohistochemical markers p16 and p53, MMR, and several traditional prognosticators such as the T and N stage, histopathological grade, lymphatic, vascular, and perineural invasion. In our retrospective study, we analyzed all specimens of pSCC from 3 centers in the Czech Republic from the period of 2002-2022.
Histopathologically were determined staging, grading, lymphatic, venous, and perineural invasion, and tumor budding – invasion of tumor cells around the tumor mass. The number of tumor buds (single tumor cell or cluster of ⋖5 tumor cells) was counted in one power field with 20x magnification selecting the area with the highest budding. In cases of discordant counts from both pathologists, an arithmetic mean value was used. The cases were sorted according to the bud number using a three-tiered system [low grade budding (0-4), moderate budding (5-9), and high grade budding (⋗9)] and also using a two-tiered system [low-grade budding (0-4), high grade budding (≥5)]. For the evaluation of intratumoral and peritumoral lymphocytic infiltration, we adopted the scheme widely used in malignant melanoma8: the lymphocytic infiltrate was classified using hematoxylin-eosin stained slides as brisk (present throughout the SCC invasive component or infiltrating continuously the entire base of the invasive tumor), non-brisk (lymphocytes present in one or more foci of the invasive SCC), or absent (no lymphocytes in contact with the invasive tumor, but some may be present in the perivascular or fibrotic areas). To objectively evaluate the number of TILs we counted them manually, using the CaseViewer 3DHISTECH software as the number of positive cells per square millimeter of the slide area. To classify the cohort according to the TIL number, the immunoscore (IS) concept developed by Galon et al. for colorectal carcinoma was adopted.9
Immunohistochemically, p53, p16 expression, and mismatch-repair (MMR) status were evaluated. The expression of p53 was evaluated as ‘mutated‘; when the tumor tissue showed diffuse strong nuclear positivity or complete negativity with heterogeneous (wild-type) expression in the stromal cells; the cases with heterogenous nuclear staining of tumor cells were labeled ‘wild-type‘. The expression of p16 was labeled ‘block-positive‘ (cytoplasmic and nuclear staining in ⋗74% of viable tumor cells), ‘non-block-positive‘ (⋖75% of viable tumor cells), and ‘negative‘. Concerning the MMR status, tumors with any apparent nuclear staining with MSH2, MSH6, PMS2, and MLH1 were considered MMR-proficient. Tumors with an obvious loss of nuclear staining of anti-MMR antibodies with positivity in the stromal cells and lymphocytes were considered MMR-deficient.
Follow-up data was gathered from patient medical records or exported from the Czech National Oncological Registry. Overall survival (OS) and cancer-specific survival (CSS) were calculated from the date of surgery to the date of recorded death or to the last known follow-up date (censoring). The stage of the tumors was assigned based on medical records. Stage I-IV was assigned in accordance with TNM Classification and Union for International Cancer Control (UICC).10
In a studied period, 152 patients with an average of 65.52 years (range 31.4 – 91.8 years) were included in the study and the results showed the following: Tumor budding (≥5 tumor buds/20x power field) and non-brisk/absent lymphocytic infiltrate are significant negative predictors of both OS and CSS in pSCC, while low immunoscore is a significant marker of shorter OS but not CSS. In our opinion, budding and subjectively evaluated lymphocytic infiltrate represent cheap and effective parameters to be reported in standard histopathological reports, which enables better risk stratification of patients suffering from pSCC. The negative prognostic significance of previously described parameters (lymphatic, venous, and perineural invasion, regional lymph node metastasis, and the p53 mutated profile) was confirmed in our study. Grade, histological subtype, and HPV status (determined by p16 immunohistochemistry) did not show any prognostic significance.
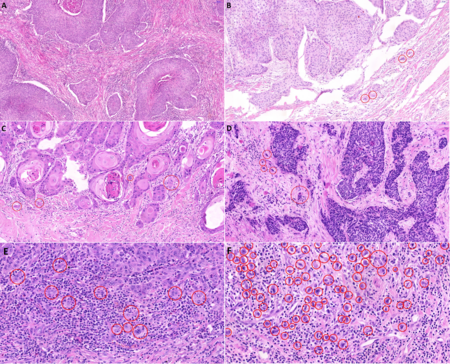
Figure 1 - Histological slide scans showing penile squamous cell carcinoma invasion front, areas with 20x magnification. A - no budding, B, C - low grade budding (4 buds/20x power field), D- moderate/high grade budding with 5 buds/20x power field in two/three-tiered grading system, respectively, E - high grade budding in high power field (11buds/40x power field), F - high grade budding reaching >70 buds in high power field. HE stain, 40x.
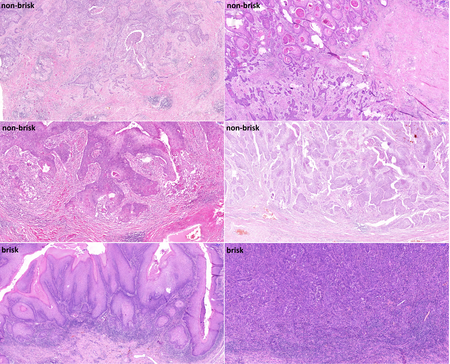
Figure 2 - Histological slide scans documenting penile squamous cell carcinoma with various lymphocytic density evaluated by two pathologists (reaching a consensus) as non-brisk / brisk lymphocytic infiltrate. HE stain, 5x.
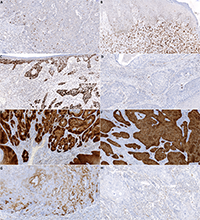
Figure 3 - Histological slide scans showing immunohistochemical reactions of penile squamous cell carcinoma with anti-p53 (A-D) and anti-p16 (E-H) antibodies. A - variable (wild-type) result showing a spectrum of nuclear negativity to weak – moderate – strong positivity in the tumor cells, B – diffuse strong nuclear p53 positivity in the tumor cells, with wild-type expression in the adjacent non-neoplastic epithelium (mutated pattern), C - diffuse strong nuclear p53 positivity in the invasive carcinoma cells and the precancerous lesion (differentiated/simple penile intraepithelial lesion) in the surface epithelium (mutated pattern), D – complete p53 negativity in the tumor cells (mutated pattern) and weak (wild-type) reaction in the non-neoplastic stromal cells and lymphocytes, E - diffuse strong cytoplasmic and nuclear (block) p16 positivity in keratinizing squamous carcinoma, F - diffuse strong cytoplasmic and nuclear (block) p16 positivity in basaloid squamous carcinoma, G – non-block (weak and focal) p16 positivity in <75% of viable tumor cells of invasive squamous carcinoma, H – p16 negative keratinizing squamous carcinoma. Magnification 20x.
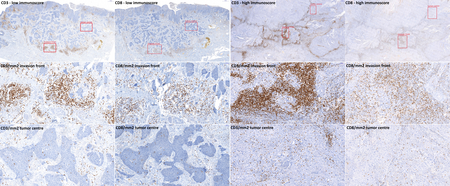
Figure 4 - Histological slide scans showing immunohistochemical reactions of tumor infiltrating T-cells within penile squamous cell carcinoma with anti-CD3 and anti-CD8 antibodies. To assess the immunoscore, CD3+ and CD8+ lymphocytes in an area of 1 mm2 were counted using the CaseViewer software in both tumor centre and invasion front. Red squares show an area of 1 mm2.
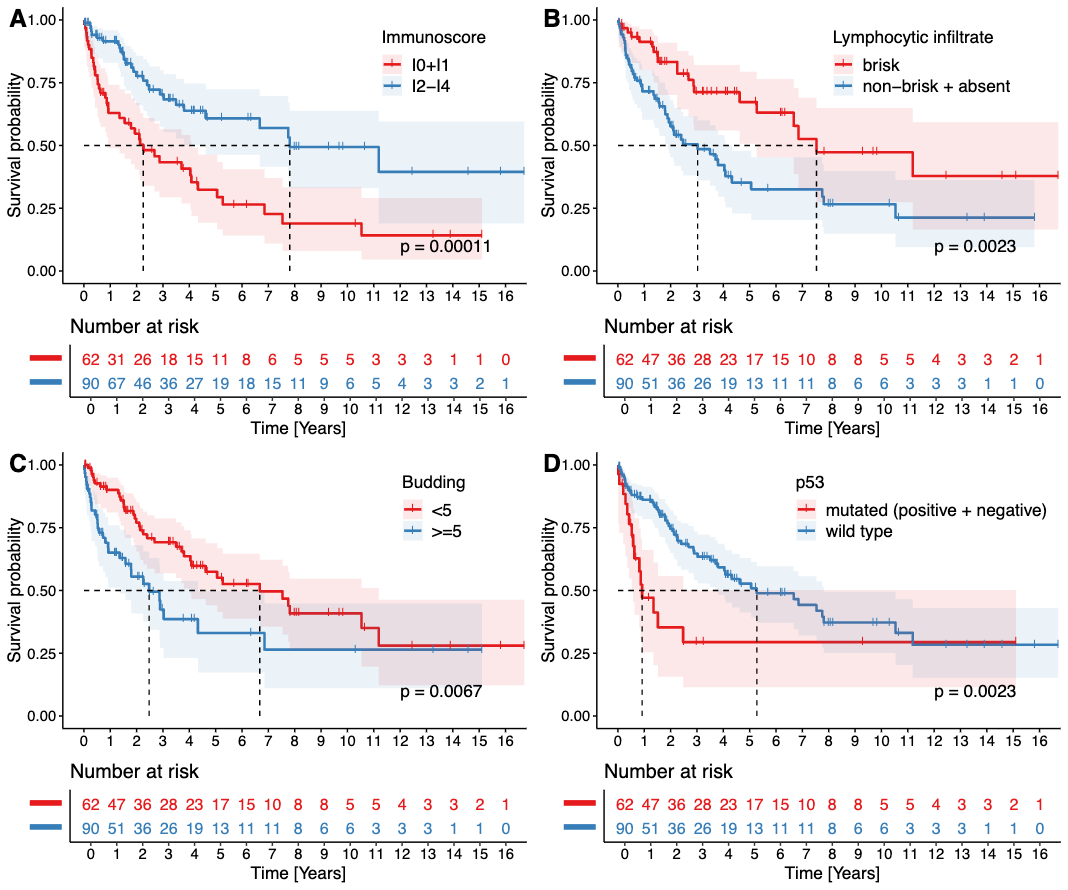
Figure 5 – Kaplan-Meier curves showing the overall survival analysis of the main significant variables. Note the significant negative prognostic impact of low immunoscore (A), non-brisk or absent lymphocytic infiltrate (B), high grade budding in two-tiered (C) system, and p53 mutated pattern (D).
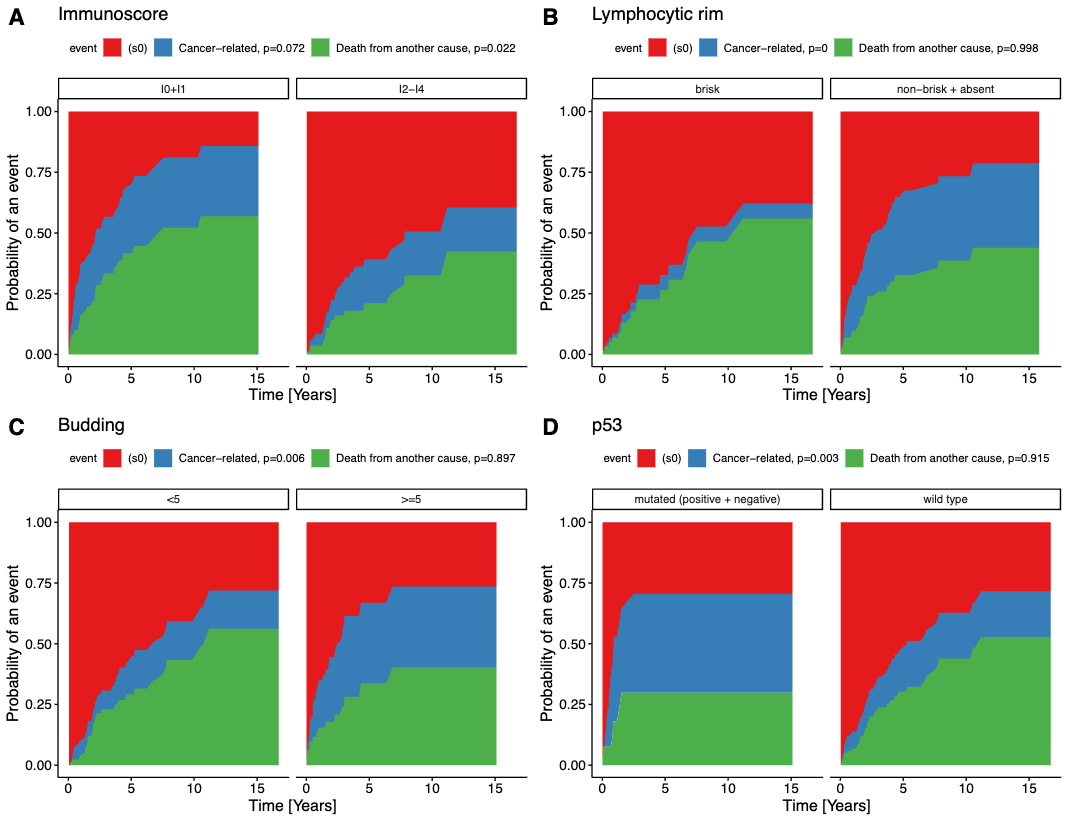
Figure 6 - Aalen-Johansen cumulative incidence curves showing cancer-specific survival. Note the difference between the blue area documenting the ratio of cancer-related deaths and deaths of other/unknown causes. There is a significant impact of lymphocytic infiltrate (B), high-grade budding in the two-tiered system (C), p53 mutated pattern (D). A low immunoscore (A) was a significant predictor of cancer-unrelated death, but not of cancer-specific death.
Written by: Jan Hrudka,1* Zuzana Prouzová,1,2 Michaela Bártů,2 Jan Hojný,2 David Čapka,3 Nicolette Zavillová,4 Radoslav Matěj,1,2,5 Petr Waldauf6
- Department of Pathology, 3rd Faculty of Medicine, Charles University, University Hospital Kralovske Vinohrady, Prague, Czech Republic.
- Department of Pathology, 1st Faculty of Medicine, Charles University, General University Hospital, Prague, Czech Republic.
- Department of Urology, 3rd Faculty of Medicine, Charles University, University Hospital Kralovske Vinohrady, Prague, Czech Republic.
- Department of Urology, 3rd Faculty of Medicine of Charles University, Thomayer University Hospital, Prague, Czech Republic.
- Department of Pathology and Molecular Medicine, 3rd Faculty of Medicine, Charles University, Thomayer University Hospital, Prague, Czech Republic.
- Department of Anaesthesia and Intensive Care Medicine, 3rd Faculty of Medicine, Charles University, University Hospital Kralovske Vinohrady, Prague, Czech Republic.
References:
- Hakenberg OW, Compérat EM, Minhas S, et al. EAU guidelines on penile cancer: 2014 update. Eur Urol. 2015;67(1):142-150.
- Cubilla AL, Velazquez EF, Amin MB, Epstein J, Berney DM, Corbishley CM. The World Health Organisation 2016 classification of penile carcinomas: a review and update from the International Society of Urological Pathology expert-driven recommendations. Histopathology, 2018; 72: 893-904.
- May M, Burger M, Otto W, Hakenberg OW, Wieland WF, May D, Hofstädter F, Götz S, Niessl N, Fritsche HM, Birnkammer K, Gilfrich C, Peter J, Jain A, Koch S, Lebentrau S, Riedmiller H, Rössler W, Denzinger S, Brookman-May S, Gunia S. Ki-67, mini-chromosome maintenance 2 protein (MCM2) and geminin have no independent prognostic relevance for cancer-specific survival in surgically treated squamous cell carcinoma of the penis. BJU Int. 2013 Aug;112(4):E383-90.
- Buonerba C, Pagliuca M, Vitrone FM, et al. Immunotherapy for penile cancer. Future Sci OA. 2017;3(3):FSO195. Published 2017 May 2.
- Ottenhof SR, Djajadiningrat RS, Thygesen HH, et al. The Prognostic Value of Immune Factors in the Tumor Microenvironment of Penile Squamous Cell Carcinoma. Front Immunol. 2018;9:1253.
- Lohneis P, Boral S, Kaufmann AM, et al. Human papilloma virus status of penile squamous cell carcinoma is associated with differences in tumour-infiltrating T lymphocytes. Virchows Arch. 2015;466(3):323‐331.
- da Cunha IW, Souza MJ, da Costa WH, et al. Epithelial-mesenchymal transition (EMT) phenotype at invasion front of squamous cell carcinoma of the penis influences oncological outcomes. Urol Oncol. 2016;34(10):433.e19‐433.e4.33E26.
- Clark WH Jr, Elder DE, Guerry D 4th, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81(24):1893-904.
- Galon J, Mlecnik B, Bindea G, et al. Towards the introduction of the "Immunoscore" in the classification of malignant tumours. J Pathol. 2014;232(2):199-209.
- Brierley, J.D., Gospodarowicz, M.K., Wittekind, C. TNM Classification of Malignant Tumors, 8th edition. Wiley Blackwell, 2017.


