Janssen to Present 14 Abstracts in Prostate and Urothelial Cancers at ASCO GU 2018, Including New Data on Apalutamide (ARN-509), ZYTIGA® (abiraterone acetate) and Erdafitinib
- Phase 3 SPARTAN clinical trial results for apalutamide in non-metastatic castration-resistant prostate cancer selected for Prostate Cancer Oral Abstract Session
- 10 poster presentations for ZYTIGA® (abiraterone acetate) including new analyses from the pivotal Phase 3 LATITUDE trial assessing ZYTIGA® plus prednisone and ADT in metastatic hormone-naïve prostate cancer
- Phase 2 erdafitinib data in patients with metastatic or unresectable urothelial carcinoma and FGFR alterations selected for Urothelial Cancers Rapid Fire Abstract Session
“The data, which is being presented at ASCO GU and features a number of approved and investigational compounds, reinforces our commitment to helping transform patient outcomes. We understand that every patient with cancer will face his or her own unique journey and we are committed to helping redefine that journey,” said Dr. Ivo Winiger- Candolfi, Oncology Solid Tumor Therapy Area Lead, Janssen Pharmaceutical Companies of Johnson & Johnson. “We particularly look forward to presenting results from the pivotal SPARTAN clinical trial.”Key company-sponsored data presentations include:
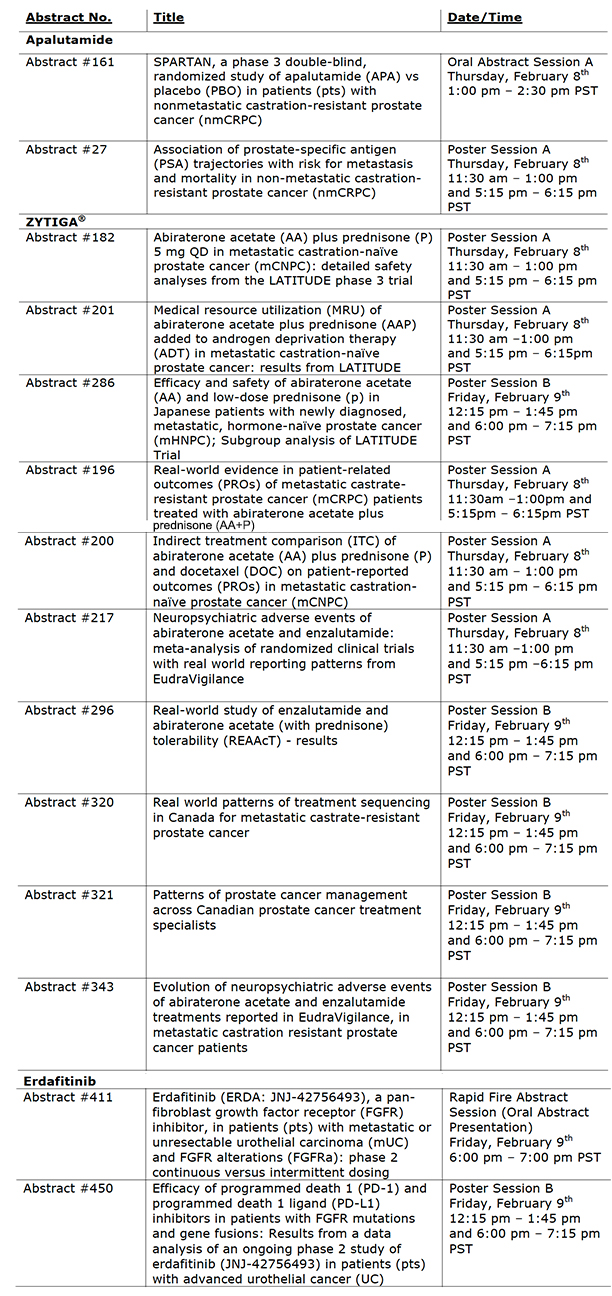
Apalutamide:
- SPARTAN, a phase 3 double-blind, randomized study of apalutamide (APA) vs placebo (PBO) in patients (pts) with non-metastatic castration-resistant prostate cancer (nmCRPC) (Abstract #161)
1:00 pm – 2:30 pm PST on Thursday, February 8th.
ZYTIGA®:
- Abiraterone acetate plus prednisone (P) 5 mg QD in metastatic castration-naïve prostate cancer (nCNPC): detailed safety analyses from the LATITUDE phase 3 trial (Abstract #182)
February 8th
- Medical resource utilization (MRU) of abiraterone acetate plus prednisone (AAP) added to androgen deprivation therapy (ADT) in metastatic castration-naïve prostate cancer: results from LATITUDE (Abstract #201)
□ This data will be presented in a Poster Presentation Session A from 11:30am-1:00pm and 5:15 pm – 6:15 pm PST on Thursday, February 8th
- Efficacy and safety of abiraterone acetate (AA) and low-dose prednisone (P) in Japanese patients with newly diagnosed, metastatic, hormone-naïve prostate cancer (mHNPC); Subgroup analysis of LATITUDE trial (Abstract #286)
Erdafitinib:
- Erdafitinib (ERDA; JNJ-42756493), a pan-fibroblast growth factor receptor (FGFR) inhibitor, in patients (pts) with metastatic or unresectable urothelial carcinoma (mUC) and FGFR alterations (FGFRa): phase 2 continuous versus intermittent dosing (Abstract #411)
PST on Friday, February 9th
A full list of company-sponsored abstracts to be presented at the meeting follows below: