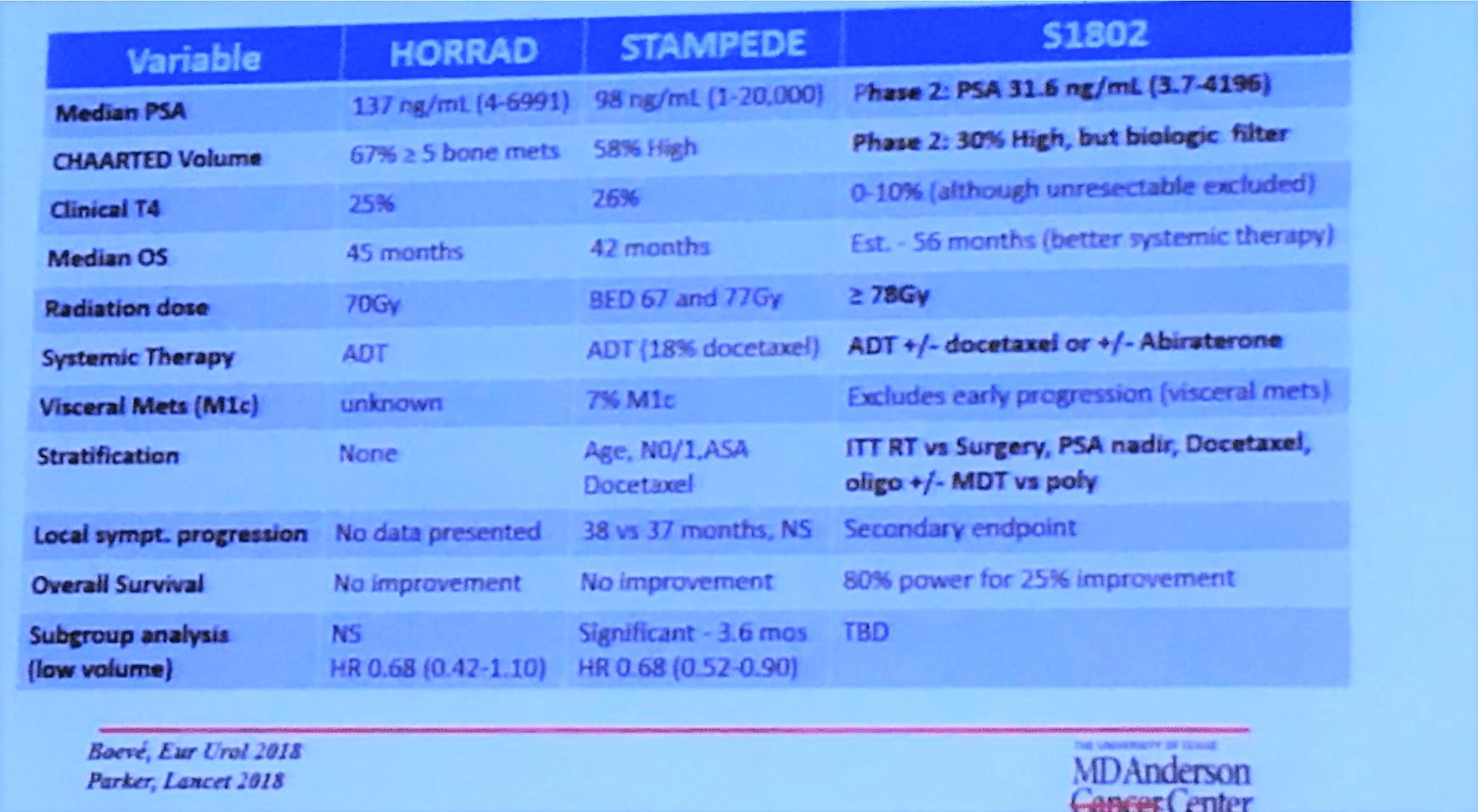
Previously, the HORRAD1 and STAMPEDE2 trials investigated whether or not there was a benefit to local therapy in the setting of metastatic disease. In the HORRAD study, patients with greater than 5 metastatic lesions were able to enroll. Hormone versus hormone therapy with radiation therapy to the primary was compared and there was an improvement in progression-free survival (PFS) in patients treated with radiation therapy, but no difference in overall survival. In the STAMPEDE trial, the standard of care versus standard of care plus radiation therapy was compared. There was no overall survival difference, but there was an improvement in failure-free survival in patients treated with radiotherapy. In the subgroup analysis, there was some evidence of benefit if there were less than 5 metastatic lesions, however, this is considered to be hypothesis generating.
Dr. Chapin is the principal investigator for the SWOG s1802 trial. Patients with greater than or equal to M1a castrate-sensitive prostate cancer will undergo standard systemic therapy and then be randomized to either receive definitive local treatment (either radiation or surgery) or continue with standard systemic therapy only. The primary endpoint is overall survival. The secondary endpoints are overall survival in patients who received standard systemic therapy plus surgical excision versus standard systemic therapy alone, comparison of the rate of symptomatic local progression between the treatment arms and progression-free survival. There is also the quality of life objectives. What is this going to add in the setting of the HORRAD and STAMPEDE trial? First, definitive treatment will be either surgery or radiation, whereas the previous two trials were just treated with radiation. Second, the dose of radiation treatment will be in line with contemporary doses which may result in a difference in survival. Finally, we now have better systemic therapy regimens which could impact survival differences potentially missed in the prior studies.
Presented by: Brian F. Chapin, MD, Associate Professor of Genitourinary Oncology, MD Anderson Cancer Center, Houston, Texas
References:
1. Boevé LMS, Hulshof MCCM, Vis AN, Zwinderman AH, Twisk JWR, Witjes WPJ, et al. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. Eur Urol. 2018.
2. Parker CC, James ND, Brawley CD, Clarke NW, Hoyle AP, Ali A, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018.
Written by: Dr. Amy H. Lim, MD, PhD, Urologic Oncology Fellow and Ashish M. Kamat, MD, (@UroDocAsh), Professor of Urologic Oncology & Cancer Research, MD Anderson Cancer Center, Houston, TX at the 13th Update on the Management of Genitourinary Malignancies, The University of Texas (MDACC - MD Anderson Cancer Center) November 9-10, 2018, Dan L. Duncan Building, Houston, TX
If interested in enrolling patients in this trial, email or .


