Background: Pivotal trials of AAP for patients with mCRPC enrolled few B pts, a population with a higher mortality from prostate cancer. Retrospective data suggests B pts may have higher PSA response rates than W pts treated with AAP for mCRPC. Therefore, we prospectively investigated AAP in B vs. W pts with mCRPC.
Methods: Abi Race (NCT01940276) is a prospective, multicenter, parallel group study of AP in 100 men (50 B, 50 W) with mCRPC, self-identified by race. All pts received AA 1000 mg/D and P 10 mg/D (AAP) until disease progression or unacceptable adverse events (AE). The primary objective was radiographic progression-free survival (rPFs); key secondary endpoints include PSA kinetics and safety. Exploratory analyses include SNP, metabolomics and hormonal differences by race.
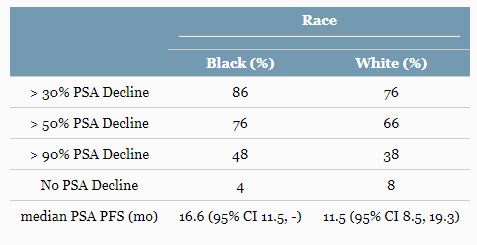
Results: Baseline characteristics among pts were similar. The median rPFS for B and W pts was 16.8 months (mo) in each. However, PSA PFS varied by race; median PSA PFS for B and W pts were 16.6 and 11.5 mo [Table]. B pts also had numerically higher rates of ≥30%, ≥50% and ≥90% PSA decline [Table]. AEs were similar in frequency and severity by race including hypertension (42 vs 40%); however, fatigue was higher in W pts (40 vs 26%), and hypokalemia was higher in B pts (36 vs 18%). SNP profiling revealed differences in key genes involved in androgen metabolism and transport.
Conclusions: This is the first prospective multicenter study by the race of secondary hormonal therapy in mCRPC. B pts may have greater and more durable PSA response to AAP than W pts. SNP patterns vary by race and will be evaluated for prognostic significance. Further prospective studies in B pts are possible and needed to understand the impact of racial determinants on the outcome of new hormonal regimens. Clinical trial information: NCT01940276.
PSA response outcomes.
Journal of Clinical Oncology. 2018, June 07 [Epub ahead of print]
Daniel J. George1, Elisabeth I. Heath2, A. Oliver Sartor3, Guru Sonpavde4, William R. Berry5, Patrick Healy6, Carolyn Winters1, Colleen Riggan6, Monika Anand1, Julie Kephart6, Matthew I. Milowsky7, Mark T. Fleming8, K. C. Balaji9, Tian Zhang10, Rhonda L. Bitting11, Michael Roger Harrison6, Megan Ann McNamara12, Jennifer A. Freedman13, Susan Halabi13, Andrew J. Armstrong6, Deborah Bruner14
1. Duke University, Durham, North Carolina
2. Barbara Ann Karmanos Cancer Institute, Wayne State University, Detroit, Michigan
3. Tulane Medical School, New Orleans, Louisiana
4. Dana-Farber Cancer Institute, Boston, Massachusetts
5. Duke University School of Medicine, Raleigh, North Carolina
6. Duke Cancer Institute, Duke University Medical Center, Durham, North Carolina
7. UNC School of Medicine, Chapel Hill, North Carolina
8. Virginia Oncology Associates, US Oncology Research, Norfolk, Virginia
9. Wake Forest University Health Sciences, Winston Salem, North Carolina
10. Duke University Medical Center, Durham, North Carolina
11. Internal Medicine, Section on Hematology and Oncology, Winston Salem, North Carolina
12. Duke University Medical Center, Mornsville, North Carolina 13. Duke University Medical Center, Durham, North Carolina
14. Winship Cancer Institute at Emory University, Atlanta, Georgia
DOI: 10.1200/JCO.2018.36.18_suppl.LBA5009 Journal of Clinical Oncology 36, no. 18_suppl