METHODS: We used the DFCI CRIS database to identify cohorts of patients (pts) who developed mCRPC between 2004-2007 (cohort A) and 2010-2013 (cohort B). Therapies for mCRPC in each cohort were annotated. Given the median follow-up (FU) was 10.6 years (yrs) in cohort A and 4.6 yrs in cohort B, we evaluated OS, defined as the time from mCRPC per PCWG3 criteria to death from all causes or last follow-up visit within 5 yrs (truncated OS). Kaplan-Meier method estimated the time to events distribution with median (95% CI). Cox proportional hazards model evaluated the effects of treatment groups on disease outcomes with estimates of hazard ratio (95% CI).
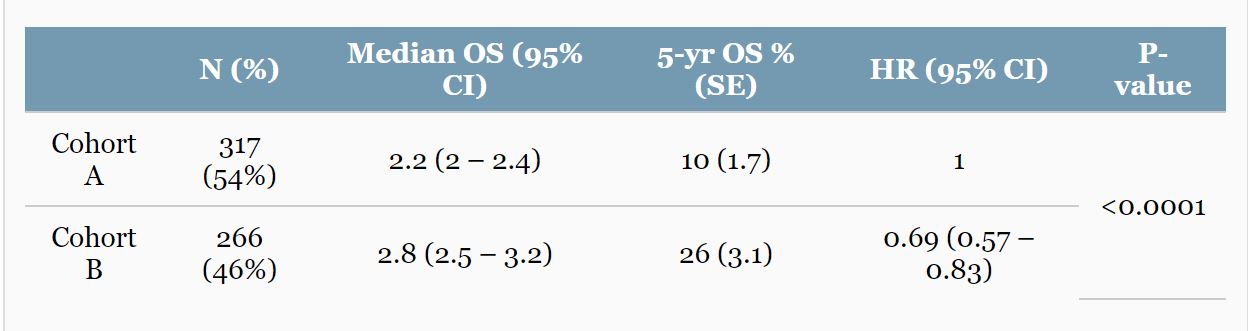
RESULTS: Of the 583 pts identified, 317 (54%) were in cohort A and 266 (46%) in cohort B. pts in cohort B had a significantly longer median OS (p<0.001), a 5-yr OS of 26% vs. 10%, and a 31% reduced risk of death compared to cohort A (HR=0.69; 95% CI, 0.57-0.83) (see Table). On multivariable analysis, adjusting for prior local Rx, ECOG PS, and the number of agents received, longer OS is confirmed associated with cohort B vs. A and also with ECOG PS status 0 vs. 1, a number of agents received 5-12 vs. ≤3.
CONCLUSIONS: Using the DFCI prostate cancer database, therapies approved for mCRPC since 2010 showed a modest impact on OS, with a median improvement of 6 months. There was a more substantial effect on long-term survivors with a 2.6 fold increase of 5-yr OS.
Journal of Clinical Oncology 36, no. 6; [Epub February 26, 2018]
Edoardo Francini, Kathryn P. Gray, Grace Shaw, Carolyn Evan, Anis Hamid, Caitlin E Perry, Philip W. Kantoff, Mary-Ellen Taplin, Christopher Sweeney
Sapienza University of Rome, Rome, Italy; Dana-Farber Cancer Institute, Boston, MA; Lank Center for Genitourinary Oncology, Dana-Farber Cancer Institute, Boston, MA; Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY
Journal of Clinical Oncology 36, no. 6_suppl (February 2018) 203-203; DOI: 10.1200/JCO.2018.36.6_suppl.203


