PCa occurs mainly in older men, nearly two-thirds are diagnosed in men aged 65 or older. The mean age at the time of diagnosis is about 67 years.5 However, in a substantial subset of patients, the disease will be slow-growing and harmless,6 given the fact that autopsy data show histological prostate cancer in 80% of men at 80 years of age.7 This highlights one of the major issues with PCa screening and diagnosis: The risks of overdetection and overtreatment, i.e. to diagnose and invasively treat indolent cancers that may lead to reduced quality of life without increasing overall survival.
The systematic screening for PCa with prostate-specific antigen (PSA) testing over men’s lifetime leads to an estimated gain of 56 QUALY’s per 1,000 men screened (confidence interval [CI] from -21 to +121).8 However, as screening addresses a healthy population and screened men may suffer from disadvantages (overdiagnosis and overtreatment, unnecessary biopsies), screening in PCa remains controversial.
PSA is not a PCa specific marker, has a low predictive value, and blood concentration can raise due to many benign conditions such as benign prostate enlargement or inflammation.9,10 The consequences are unnecessary biopsies in 65–70% of men presenting with increased PSA in the range of 4-10 ng/ml,11 a potentially harmful procedure.12
Current guidelines recommend besides biomarker-based tests or risk calculators the use of multiparametric magnetic resonance imaging (mpMRI) to support biopsy decisions in subjects with inconclusive PSA results in the 2-10 ng/ml range.13 However, the use of mpMRI for the diagnosis of PCa is frequently reported as indeterminate, often leading to further examinations or unneeded biopsies with associated patient anxiety and risks. In addition, mpMRI is considered to be costly or the access to prostate MRI and radiologists is limited.
Proclarix®, a blood-based biomarker test has been developed to improve the biopsy decision algorithm. Proclarix® combines thrombospondin-1 (THBS1), cathepsin D (CTSD), total PSA (tPSA), free PSA (fPSA), and patient age to compute a risk score. Proclarix®, as an easy to use simple protein-based blood test, can be done with the same sample as the PSA test. No additional intervention is required with results becoming quickly available. Any local diagnostic laboratory can easily add this affordable multiparametric test to the existing infrastructure.
The novel biomarkers THBS1 and CTSD were originally discovered using a genetics-guided biomarker discovery approach focusing on the PI3K/PTEN cancer pathway which plays a dominant role in PCa development and progression (Figure 1).14,15
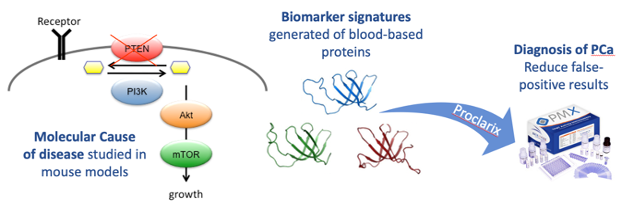
Figure 1. Translational approach for biomarker discovery and validation workflow. Cancer genetics-guided discovery of protein biomarker signatures in serum that correlate with diagnosis, prognosis, and therapy response of prostate cancer, tested in human serum from patients harboring localized prostate cancer and control patients.
Using a PTEN knock-out mouse model and mass-spectrometry based proteomics in murine tissue and human serum including a glycoprotein enrichment technology,16 several proteins directly linked to the molecular cause of cancer and therefore correlating to the disease status in the prostate were identified (Figure 2).17

Figure 2. Translational approach for biomarker discovery and validation workflow. (a) Candidate biomarkers are discovered using a genetic mouse model and (b) tested in human serum from patients harboring localized prostate cancer and control patients.
Following discovery and identification, the diagnostic potential was evaluated in several studies (Figure 3).18,19

Figure 3. Receiver operating characteristic (ROC) curves depicting the accuracy in predicting prostate cancer. In predicting prostate cancer the combination of free PSA (%fPSA), age, CTSD, THBS1 was significantly better than %fPSA (p<0.001).
The analytical and clinical performances of Proclarix® have been established with retrospective data20,21 and the test is CE-marked, with sensitivity and negative predictive value (NPV) for clinically significant prostate cancer (csPCa) defined as Gleason grade (GG) ≥2 of 90% and 95%, respectively. Furthermore, recent results of Proclarix® showed a strong correlation with the mpMRI-based Likert score, a scoring system used to evaluate prostate mpMRI, indicating that Proclarix® could accurately discriminate among patients within the indeterminate (Likert score 3) mpMRI category.22 In this study, Proclarix® had an NPV of 100%, at 100% sensitivity and a specificity of 34%, indicating that in men with an indeterminate mpMRI-result, Proclarix® could allow one-third to safely avoid biopsies without missing any csPCa. Using the Proclarix® density could even further increase the specificity and thus save unneeded interventions.
The multicentre PROspective Prostate biOmarker Study (PROPOSe) (NCT03565289, AUO Study AP 98/18) was initiated to confirm the previously established diagnostic accuracy of Proclarix® in everyday practice. Ten clinical sites prospectively enrolled 457 men presenting for prostate biopsy with PSA between 2-10 ng/ml, normal DRE and prostate volume ≥35 cm³ (Figure 4).

Figure 4. Participating clinical centers in the PROPOSe study. Ten clinical centers from three countries including Austria (1), Denmark (1), and Germany (8) prospectively collected serum samples.
This prospective study confirmed Proclarix® performance as established during CE validation in clinical routine use. Proclarix® detected clinically significant cancer with high sensitivity above 90% and reliably ruled out patients with no or indolent cancer with a negative predictive value greater than 90%. When the biopsy performed was guided by mpMRI both sensitivity (97%) and NPV (96%) were even higher. Importantly, Proclarix® was significantly superior to the current clinical standard percent free PSA in ruling out unneeded biopsies (22% vs. 14%) and the primary study endpoint was met (p-value < 0.005).
Prof. Dr. med. Thomas Steuber from the renowned Martini-Klinik Hamburg and principal investigator of the study stated: “In a routine use setting, Proclarix® accurately discriminated clinically significant PCa from no or indolent PCa. This study provides strong support for the use of Proclarix® in routine practice to improve the biopsy decision algorithm.”
Co-investigator Prof. Dr. med. Carsten Ohlmann from the Johanniter Krankenhaus Bonn added: “Proclarix® is a novel blood-based test with the potential to accurately rule out no or insignificant cancer, and therefore to reduce the number of unneeded biopsies.”
Written by: Oliver Straub, PhD, Medical Advisor, Proteomedix AG, Zürich, Switzerland, and Thomas Steuber, MD, Martini-Klinik, University Hospital Hamburg-Eppendorf, Hamburg, Germany
References:
- Ferlay, Jacques, M. Colombet, I. Soerjomataram, C. Mathers, D. M. Parkin, M. Piñeros, A. Znaor, and F. Bray. "Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods." International journal of cancer 144, no. 8 (2019): 1941-1953.
- Baade, Peter D., Danny R. Youlden, and Lauren J. Krnjacki. "International epidemiology of prostate cancer: geographical distribution and secular trends." Molecular nutrition & food research 53, no. 2 (2009): 171-184.
- Jemal, Ahmedin, Taylor Murray, Elizabeth Ward, Alicia Samuels, Ram C. Tiwari, Asma Ghafoor, Eric J. Feuer, and Michael J. Thun. "Cancer statistics, 2005." CA: a cancer journal for clinicians 55, no. 1 (2005): 10-30.
- Data extracted from Globocan 2020 by the International Agency for Research on Cancer. https://gco.iarc.fr/today/data/factsheets/cancers/27-Prostate-fact-sheet.pdf (accessed January 13, 2021).
- What are the key statistics about prostate cancer? Available from: http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-key-statistics n.d. http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-key-statistics.
- Rider, Jennifer R., Fredrik Sandin, Ove Andrén, Peter Wiklund, Jonas Hugosson, and Pär Stattin. "Long-term outcomes among noncuratively treated men according to prostate cancer risk category in a nationwide, population-based study." European urology 63, no. 1 (2013): 88-96.
- Jemal, Ahmedin, Rebecca Siegel, Elizabeth Ward, Yongping Hao, Jiaquan Xu, Taylor Murray, and Michael J. Thun. "Cancer statistics, 2008." CA: a cancer journal for clinicians 58, no. 2 (2008): 71-96.
- Heijnsdijk, Eveline AM, Elisabeth M. Wever, Anssi Auvinen, Jonas Hugosson, Stefano Ciatto, Vera Nelen, Maciej Kwiatkowski et al. "Quality-of-life effects of prostate-specific antigen screening." New England Journal of Medicine 367, no. 7 (2012): 595-605.
- Hedelin, Hans, Niklas Johansson, and Peter Ströberg. "Relationship between benign prostatic hyperplasia and lower urinary tract symptoms and correlation between prostate volume and serum prostate-specific antigen in clinical routine." Scandinavian journal of urology and nephrology 39, no. 2 (2005): 154-159.
- Simardi, Lucila Heloisa, Marcos Tobias-MacHado, Guilherme Tommasi Kappaz, Patricia Taschner Goldenstein, Jeannette M. Potts, and Eric Roger Wroclawski. "Influence of asymptomatic histologic prostatitis on serum prostate-specific antigen: a prospective study." Urology 64, no. 6 (2004): 1098-1101.
- Hendriks, R. J., I. M. Van Oort, and J. A. Schalken. "Blood-based and urinary prostate cancer biomarkers: a review and comparison of novel biomarkers for detection and treatment decisions." Prostate cancer and prostatic diseases 20, no. 1 (2017): 12-19.
- Loeb, Stacy, Annelies Vellekoop, Hashim U. Ahmed, James Catto, Mark Emberton, Robert Nam, Derek J. Rosario, Vincenzo Scattoni, and Yair Lotan. "Systematic review of complications of prostate biopsy." European urology 64, no. 6 (2013): 876-892.
- Mottet N, Cornford P, Bergh RCN van den, Briers E, Santis MD, Fanti S, et al. EAU-EANM-ESUR-ESTRO-SIOG Guidelines on Prostate Cancer 2019. Arnhem, The Netherlands: EAU Guidelines Office; n.d.
- Sansal, Isabelle, and William R. Sellers. "The biology and clinical relevance of the PTEN tumor suppressor pathway." Journal of clinical oncology 22, no. 14 (2004): 2954-2963.
- POURMAND, GH R., AR ABEDI, AAR MEHRSAI, A. AHMADI, AA ZIAEI, HR SAADATI, and ALAVI H. AFSHIN. "Role of PTEN gene in progression of prostate cancer." (2007): 95-100.
- Schiess, Ralph, Bernd Wollscheid, and Ruedi Aebersold. "Targeted proteomic strategy for clinical biomarker discovery." Molecular oncology 3, no. 1 (2009): 33-44.
- Cima, Igor, Ralph Schiess, Peter Wild, Martin Kaelin, Peter Schüffler, Vinzenz Lange, Paola Picotti et al. "Cancer genetics-guided discovery of serum biomarker signatures for diagnosis and prognosis of prostate cancer." Proceedings of the National Academy of Sciences 108, no. 8 (2011): 3342-3347.
- Endt, Kathrin, Jens Goepfert, Aurelius Omlin, Alcibiade Athanasiou, Pierre Tennstedt, Anna Guenther, Maurizio Rainisio et al. "Development and clinical testing of individual immunoassays for the quantification of serum glycoproteins to diagnose prostate cancer." PLoS One 12, no. 8 (2017): e0181557.
- Steuber, Thomas, Pierre Tennstedt, Annalisa Macagno, Alcibiade Athanasiou, Anja Wittig, Ramy Huber, Bruno Golding, Ralph Schiess, and Silke Gillessen. "Thrombospondin 1 and cathepsin D improve prostate cancer diagnosis by avoiding potentially unnecessary prostate biopsies." BJU international 123, no. 5 (2019): 826.
- Macagno, Annalisa, Alcibiade Athanasiou, Anja Wittig, Ramy Huber, Stephan Weber, Thomas Keller, Martin Rhiel, Bruno Golding, and Ralph Schiess. "Analytical performance of thrombospondin-1 and cathepsin D immunoassays part of a novel CE-IVD marked test as an aid in the diagnosis of prostate cancer." PLoS One 15, no. 5 (2020): e0233442.
- Klocker, Helmut, Bruno Golding, Stephan Weber, Eberhard Steiner, Pierre Tennstedt, Thomas Keller, Ralph Schiess, Silke Gillessen, Wolfgang Horninger, and Thomas Steuber. "Development and validation of a novel multivariate risk score to guide biopsy decision for the diagnosis of clinically significant prostate cancer." BJUI Compass (2020).
- Pye, Hayley, Hashim Ahmed, Susan Heavey, Urszula Stopka-Farooqui, Edward Johnston, Ralph Schiess, Silke Gillessen, Shonit Punwani, Mark Emberton, and Hayley Whitaker. "Evaluation of Proclarix, a prostate cancer risk score, used together with magnetic resonance imaging for the diagnosis of clinically significant prostate cancer." (2020): 278-278.
Read the Abstract


