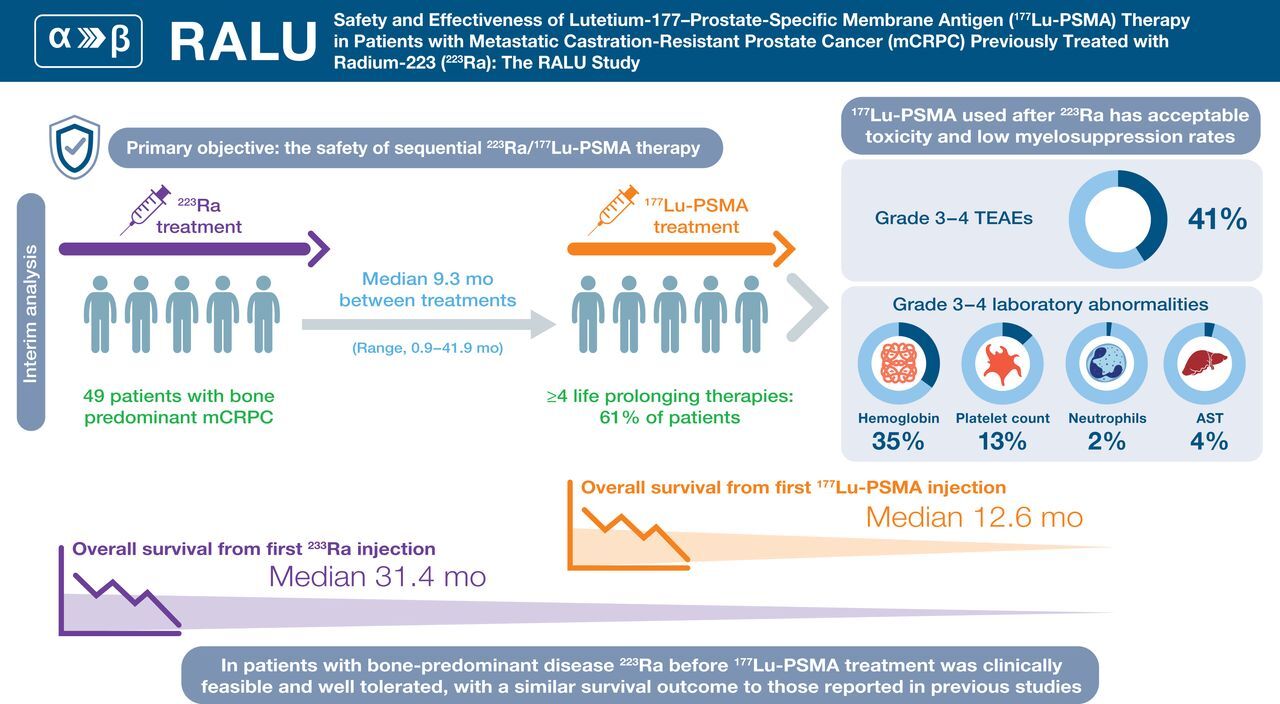
This pre-planned, interim, retrospective analysis investigated safety and survival outcomes with lutetium-177-PSMA (177Lu-PSMA) in patients treated with prior radium-223 (223Ra).
Forty-nine patients were evaluated. Patients received a median of 6 223Ra injections; 59% of patients received ≥4 177Lu-PSMA cycles. Most (69%) patients received ≥4 life-prolonging therapies before 177Lu-PSMA. Common Terminology Criteria for Adverse Events Grade 3-4 treatment-emergent adverse events during 177Lu-PSMA therapy and a 30-day follow-up period included anemia (18%) and thrombocytopenia (2%). Median overall survival was 12.6 (95% CI 8.8-16.1) and 31.4 months (95% CI 25.7-37.6) from starting 177Lu-PSMA or 223Ra, respectively.
177Lu-PSMA treatment was well tolerated in patients who had received prior 223Ra. 223Ra use before 177Lu-PSMA is feasible and can be considered for future assessment of optimal treatment sequence.
Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2022 Oct 27 [Epub ahead of print]
Kambiz Rahbar, Markus Essler, Kim M Pabst, Matthias Eiber, Christian Peter la Fougère, Vikas Prasad, Philipp Rassek, Ergela Hasa, Helmut Dittmann, Ralph A Bundschuh, Wolfgang Peter Fendler, Milena Kurtinecz, Anja Schmall, Frank Verholen, Alton O Sartor
University of Münster, Germany., University Hospital Bonn, Germany., University Hospital Essen, Germany., Technical University of Munich, Germany., University Hospital Tübingen, Germany., University of Ulm, Germany., University of Tübingen, Germany., University Augsburg, Germany., Bayer HealthCare Pharmaceuticals, United States., Bayer Consumer Care, Switzerland., Tulane University School of Medicine, United States.
Source: Rahbar K., Essler M., Pabst KM, et al. Safety and Survival Outcomes of Lutetium-177-Prostate-Specific Membrane Antigen Therapy in Patients with Metastatic Castration-Resistant Prostate Cancer with prior Radium-223 treatment: The RALU Study. Journal of Nuclear Medicine. April 2023. 64 (4) 574-578; DOI: https://doi.org/10.2967/jnumed.122.264456.


