We analyzed data of 753 patients with iT3 PCa on preoperative MRI who received RARP at five high-volume centers between 2012 and 2020. Patients who had missing data for covariates (preoperative PSA, Gleason grade, or stage; n=18), had cN+ (n=41) or iT4 (n=9) disease were excluded, leaving 685 patients eligible for analysis. Surgery was performed by experienced robotic surgeons using a conventional surgical approach to robot-assisted radical prostatectomy.7,8 Extended pelvic lymph node dissection was done in patients with preoperative risk for nodal involvement more than 5%.9 A nerve sparing technique was offered based on patient and cancer characteristics at diagnosis.
Our primary goal was to investigate predictors of organ-confined disease (i.e. pT2) on final pathology after RARP in patients with preoperative MRI suspicious for iT3 PCa. We first described frequencies and proportions for categorical variables, with medians and interquartile ranges (IQR) reported for continuous variables. Second, multivariable logistic regression investigated predictors of pT2 PCa. Our model included the following variables that were selected a priori: preoperative PSA (continuous), biopsy ISUP group (1 vs. 2 vs. 3 vs. 4 vs. 5), clinical T stage on DRE (T1 vs. T2-T3), prostate volume on MRI (continuous), PIRADS score of index lesion (3 vs. 4 vs. 5), seminal vesicles invasion on preoperative MRI (no vs. yes), location suspicious for T3 disease on preoperative MRI (mid/base vs. apex vs. transitional). In separate analyses, we also included the length of index lesion (continuous) that was available for 440 patients. Within-institution clustering was incorporated into our analyses using the cluster option in Stata statistical software. Finally, internal validation was performed using the leave-one-out cross validation (LOOCV). All statistical analyses were performed using Stata version 14.0 (StataCorp LP, College Station, TX, USA).
The characteristics of our cohort are described in Table 1. Median (IQR) preoperative PSA was 7.5 (5.2, 11.9) ng/ml, approximately half of our cohort had biopsy ISUP group 4-5 disease. Prostate cancer was palpable on DRE in 216 (32%) patients. Preoperative MRI was suspicious for iT3 disease on the mid-posterior part of the gland in 485 (71%) men, and PIRADS score was 5 in approximately two thirds of the patients.
Table 1. Descriptive characteristics of 685 patients with iT3 prostate cancer on preoperative MRI who received robot-assisted radical prostatectomy. All numbers are medians (interquartile range) and frequencies (proportions).
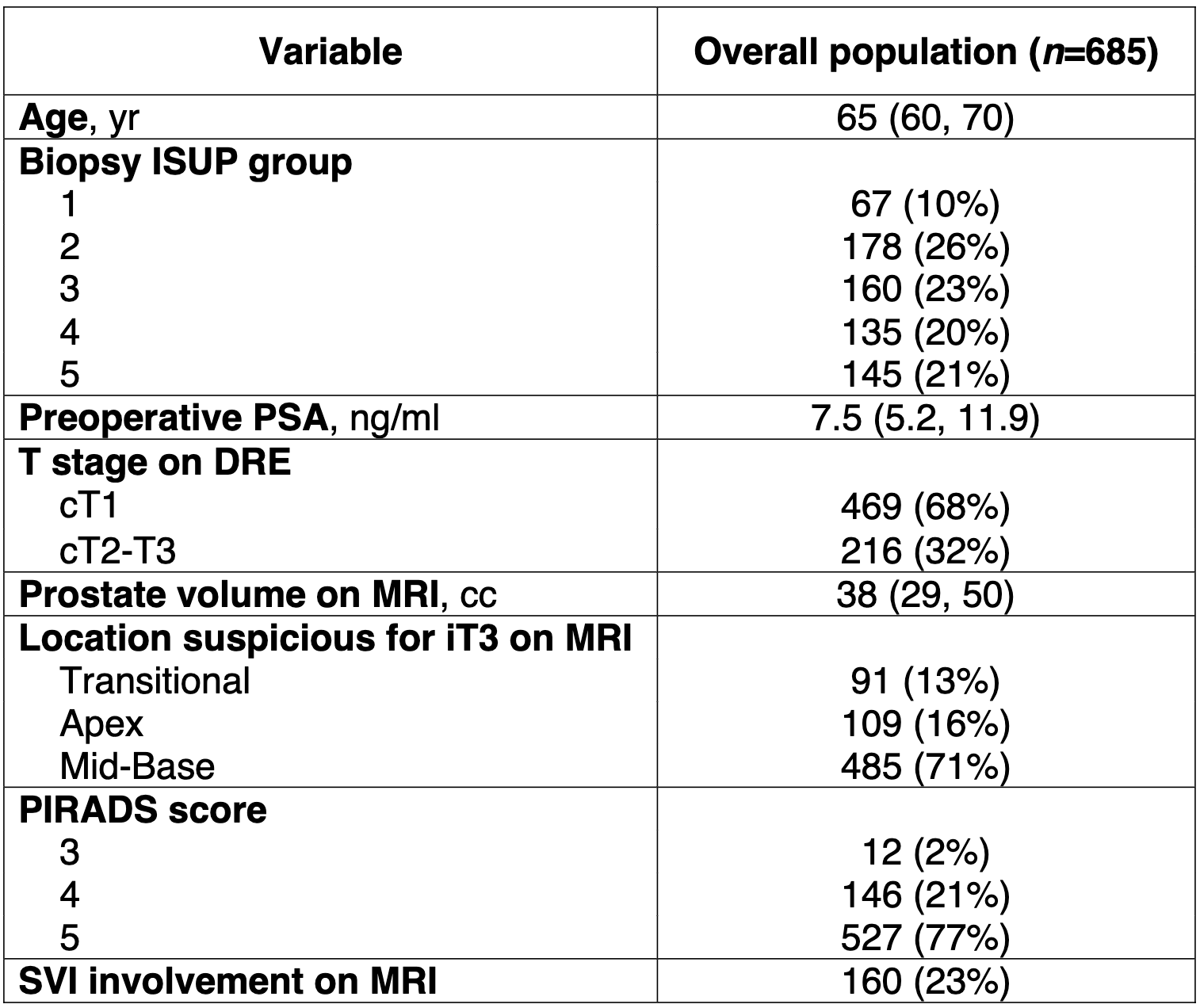
Abbreviations: ISUP: International Society of Urological Pathology; DRE: digital rectal examination; MRI: magnetic resonance imaging; PIRADS: Prostate Imaging Reporting & Data System; SVI: seminal vesicles involvement.
After surgery, a total of 192 (28%) patients had organ-confined (i.e. pT2) disease on final pathology. The role of preoperative PSA, biopsy ISUP group, clinical T stage on DRE, prostate volume on MRI, PIRADS score of index lesion, seminal vesicles invasion on preoperative MRI, location suspicious for T3 disease on preoperative MRI was investigated on multivariable logistic regression analysis. Coefficients from this model were utilized to build a nomogram for the prediction of pT2 disease on final pathology (Figure 1). The AUC after LOOCV was 73% (95% confidence interval [CI]: 69%, 77%). After the inclusion of length of index lesion to the model, the AUC after LOOCV of our model increased to 76% (95%CI: 72%, 81%).
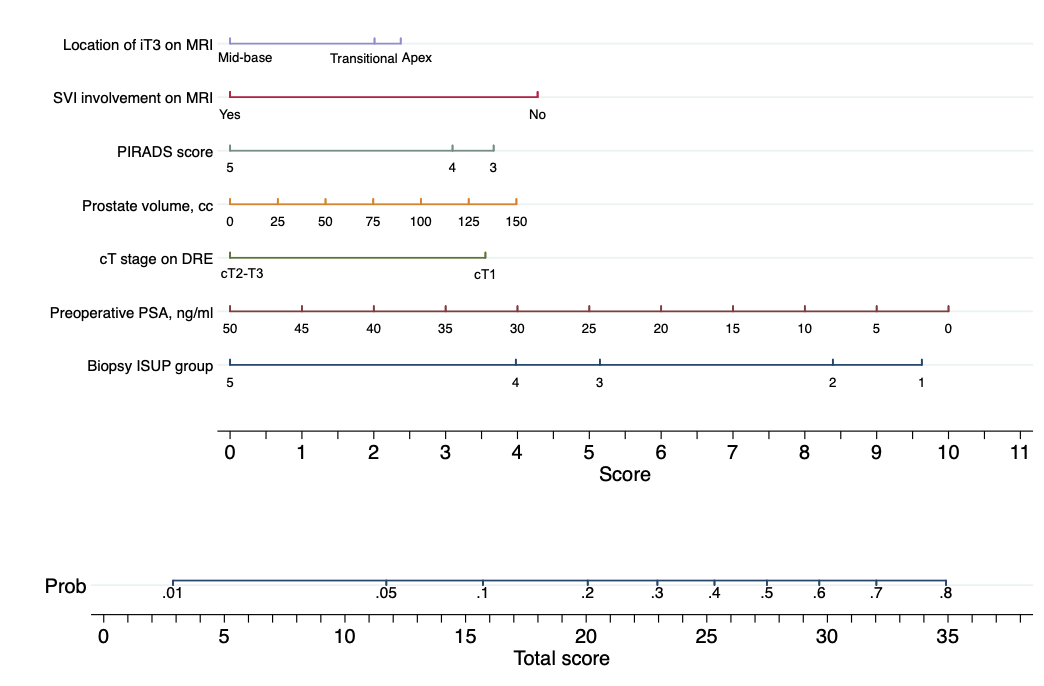
Figure 1. Nomogram for the prediction of organ-confined (i.e. pT2) disease on final pathology after robot-assisted radical prostatectomy. Instructions: Locate the patient's preoperative prostate-specific antigen (PSA) on the corresponding axis. Draw a line straight downward to the score axis to determine how many points toward the probability of pT2 the patient receives for his preoperative PSA. Repeat the process for each additional variable. Sum the points for each of the predictors. Locate the final sum on the total score axis. Draw a line straight up to find the patient's probability of having pT2 on final pathology.
We provided evidence to improve the management of men diagnosed with iT3 PCa who are candidates to RARP. In fact, it is well known that the sensitivity of MRI for local staging of prostate cancer is suboptimal6. Moreover, there are no clear recommendations on the optimal surgical strategy for patients with iT3 disease, which may translate into overtreatment. For instance, surgeons may perform a wider, more aggressive dissection in men staged iT3 on MRI who, instead, had organ-confined disease and may have received nerve-sparing surgery. In the largest series on surgically treated iT3 PCa, we demonstrated that this is the case for 1 in 3 patients. Therefore, future investigations should focus on implementing preoperative planning in this patient population. In this scenario, intra-operative use of augmented reality10 and/or confocal microscopy11 showed promising results for the prevention of positive surgical margins, and might be applicable also to the identification of extra-capsular disease together with predictive tools.
In conclusion, in the largest series of patients with iT3 PCa treated with radical prostatectomy, we showed that approximately 1 in 3 men with locally advanced PCa on preoperative MRI had pT2 disease on final pathology. We developed an easy-to-use model for the prediction of organ-confined disease on final pathology that uses preoperative variables. The nomogram-derived probability can help physicians to optimize surgical strategy, especially during the nerve-sparing, in some patients with preoperative imaging suggesting iT3 PCa.
Written by: Carlo A. Bravi1,2,3 Elio Mazzone3 Paolo Dell’Oglio4,5,6 Marcio Covas Moschovas7 Alberto Martini3 Giuseppe Rosiello3 Pietro Piazza2,8 Angelo Mottaran2,8 Marco Paciotti2,12 Luca Sarchi2,9 Stefano Puliatti2,9 Sophie Knipper2,10 Ruben De Groote1,2 Riccardo Schiavina8 Bernando Rocco9,11 Antonio Galfano4 Alberto Briganti3 Francesco Montorsi3 Vipul Patel7 Alexandre Mottrie1,2
- Department of Urology, Onze-Lieve-Vrouwziekenhuis Hospital, Aalst, Belgium
- ORSI Academy, Ghent, Belgium
- Division of Oncology/Unit of Urology; URI; IRCCS Ospedale San Raffaele, Milan, Italy
- Department of Urology, ASST Grande Ospedale Metropolitano Niguarda, Milan, Italy;
- Department of Urology, Antoni van Leeuwenhoek Hospital, The Netherlands Cancer Institute, Amsterdam, The Netherlands;
- Interventional Molecular Imaging Laboratory, Department of Radiology, Leiden University Medical Center, Leiden, The Netherlands
- AdventHealth Global Robotics Institute, Celebration, FL, USA.
- Division of Urology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy
- Department of Urology, University of Modena and Reggio Emilia, Modena, Italy
- Martini-Klinik Prostate Cancer Center, University Hospital Hamburg-Eppendorf, Hamburg, Germany
- Urological Unit, Department of Health Sciences, ASST Santi Paolo e Carlo, University of Milan, Milan, Italy
- Department of Urology, Humanitas Research Hospital, IRCCS, Rozzano, Milan, Italy
- EAU Guidelines. Edn. Presented at the EAU Annual Congress Amsterdam 2022. ISBN 978-94-92671-16-5. 2022.
- National Comprehensive Cancer Network. Prostate Cancer (Version 4.2022). https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed June 21, 2022. August 2021:1-151.
- Moris L, Cumberbatch MG, Van den Broeck T, et al. Benefits and Risks of Primary Treatments for High-risk Localized and Locally Advanced Prostate Cancer: An International Multidisciplinary Systematic Review. European Urology. March 2020:1-14. doi:10.1016/j.eururo.2020.01.033.
- Gillessen S, Attard G, Beer TM, et al. Management of Patients with Advanced Prostate Cancer: Report of the Advanced Prostate Cancer Consensus Conference 2019. European Urology. January 2020:1-40. doi:10.1016/j.eururo.2020.01.012.
- Chierigo F, Borghesi M, Wurnschimmel C, et al. Contemporary Pathological Stage Distribution After Radical Prostatectomy in North American High-Risk Prostate Cancer Patients. Clinical Genitourinary Cancer. May 2022:1-10. doi:10.1016/j.clgc.2022.04.005.
- de Rooij M, Hamoen EHJ, Witjes JA, Barentsz JO, Rovers MM. Accuracy of Magnetic Resonance Imaging for Local Staging of Prostate Cancer: A Diagnostic Meta-analysis. European Urology. July 2015:1-13. doi:10.1016/j.eururo.2015.07.029.
- Martini A, Falagario UG, Villers A, et al. Contemporary Techniques of Prostate Dissection for Robot-assisted Prostatectomy. European Urology. 2020;78(4):583-591. doi:10.1016/j.eururo.2020.07.017.
- Mazzone E, Dell'Oglio P, Rosiello G, et al. Technical Refinements in Superextended Robot-assisted Radical Prostatectomy for Locally Advanced Prostate Cancer Patients at Multiparametric Magnetic Resonance Imaging. European Urology. September 2020:1-9. doi:10.1016/j.eururo.2020.09.009.
- Gandaglia G, Fossati N, Zaffuto E, et al. Development and Internal Validation of a Novel Model to Identify the Candidates for Extended Pelvic Lymph Node Dissection in Prostate Cancer. European Urology. 2017;72(4):1-9. doi:10.1016/j.eururo.2017.03.049.
- Bianchi L, Chessa F, Angiolini A, et al. The Use of Augmented Reality to Guide the Intraoperative Frozen Section During Robot-assisted Radical Prostatectomy. European Urology. July 2021:1-9. doi:10.1016/j.eururo.2021.06.020.
- Rocco B, Sarchi L, Assumma S, et al. Digital Frozen Sections with Fluorescence Confocal Microscopy During Robot-assisted Radical Prostatectomy: Surgical Technique. European Urology. May 2021:1-6. doi:10.1016/j.eururo.2021.03.021.


