Partly, those trials utilized the miTNM framework – a system that was developed to structure reporting of molecular PET-imaging of prostate cancer. This ensures the clinical relevance of a given report to the referring urologist. The miTNM framework is part of the PROMISE initiative, which aims to standardize the reporting of PSMA-PET.4 However, there is still a significant clinical demand to not only report the status quo in a PSMA-PET but to assess response to treatment in a standardized way. This is of paramount importance not only for the clinical routine but also for clinical trials, which employ PSMA-PET to objectivize response to study treatment.
To this end, just recently the updated PROMSIE V 2.0 framework has been introduced.5 This new version is a significant advancement that not only harmonizes the miTNM score and improves its applicability for the evaluation of patients with suspected prostate cancer, but also includes a standardized reporting template to assess response to therapy. This provides a joint foundation of qualitative and quantitative PSMA-PET-derived findings, which can be used to leverage response frameworks already proposed, or of new ones still to be introduced. Briefly, by PROMISE V2, whole-body PSMA-PET image findings are summarized in a simple reporting template, which encompasses everything of interest that can be drawn from staging to response assessment. PROMISE V 2.0 will facilitate the design of prospective trials that utilize PSMA-PET to quantify the treatment effects of the study medication. In turn, the PROMISE V2 framework will further promote the use of PSMA-PET as a superior examination for patients with prostate cancer.
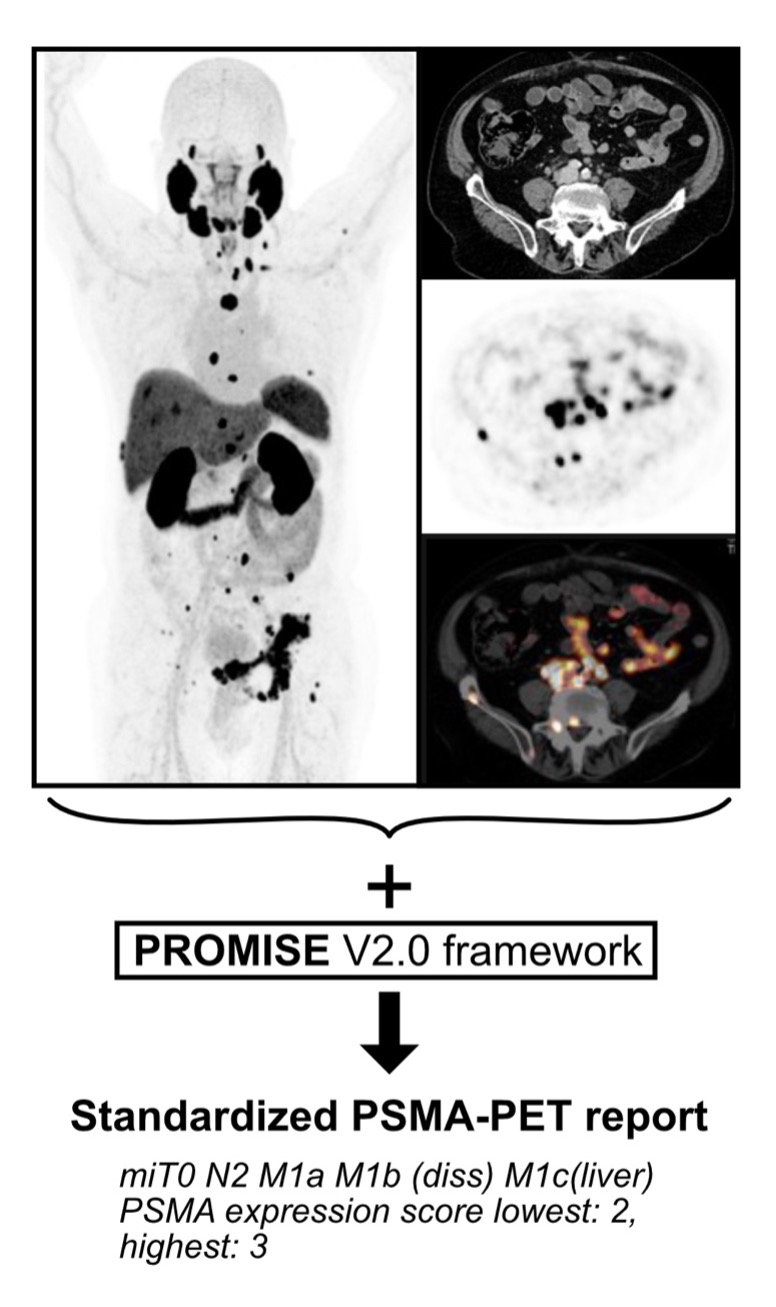
Figure: Exemplary PSMA-PET scan and standardized PROMISE report
Written by: Robert Seifert MD,1 Matthias Eiber MD, PhD,2 Wolfgang P. Fendler MD1
- Department of Nuclear Medicine, University Hospital Essen, Essen, Germany
- Bavarian Cancer Research Center (BZKF), Erlangen, Germany
References:
- Fendler WP, Calais J, Eiber M, Flavell RR, Mishoe A, Feng FY, et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol 2019;5:856–63.
- Hope TA, Eiber M, Armstrong WR, Juarez R, Murthy V, Lawhn-Heath C, et al. Diagnostic Accuracy of 68Ga-PSMA-11 PET for Pelvic Nodal Metastasis Detection Prior to Radical Prostatectomy and Pelvic Lymph Node Dissection: A Multicenter Prospective Phase 3 Imaging Trial. JAMA Oncol 2021.
- Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet (London, England) 2020;395:1208–16.
- Eiber M, Herrmann K, Calais J, Hadaschik B, Giesel FL, Hartenbach M, et al. Prostate cancer molecular imaging standardized evaluation (PROMISE): Proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J Nucl Med 2018;59:469–78.
- Seifert R, Emmett L, Rowe SP, Herrmann K, Hadaschik B, Calais J, et al. Second Version of the Prostate Cancer Molecular Imaging Standardized Evaluation Framework Including Response Evaluation for Clinical Trials (PROMISE V2). Eur Urol 2023.


