However, only approximately 5%-10% of metastatic castration-resistant prostate cancer cases have MMR mutations. Emerging data suggest that enzalutamide-treated prostate cancer patients with increased PD-L1 expression may benefit from anti-PD1/PD-L1 therapy. Unfortunately, a recent phase III IMbassador250 study reported that the addition of atezolizumab (a PD-L1 inhibitor) to enzalutamide failed to extend overall survival in patients with CRPC. However, the mechanisms underlying treatment failure remain unclear.
Prostate cancer has an immunosuppressive microenvironment and is characterized by a “cold immune phenotype.” The presence of tumor-infiltrating leukocytes is generally associated with better patient outcomes in many cancer types but not in prostate cancer. Various immunosuppressive cell subtypes are present in the tumor microenvironment of prostate cancer, including myeloid-derived suppressor cells (MDSC), which can inhibit the function of immune effector cells. Emerging studies have shown that the androgen receptor (AR) inhibits interferon gamma (IFNγ) transcription in T cells. AR blockade restores the ability of CD8+ T cells to produce IFN-γ. Enzalutamide combined with androgen deprivation therapy further enhanced the T cell response to PD-1 antibodies and prolonged survival in a mouse model of prostate cancer, suggesting that complete blocking of androgen signaling in the tumor microenvironment is essential for maximizing therapeutic benefits. Moreover, the preliminary ad-hoc analysis of the failed Phase III IMbassador250 trial revealed that interferon signaling activation in patients with CRPC favored the addition of a PD-L1 inhibitor.
Our study found that immune-related signaling pathways (interferon alpha/gamma response, T cell activation, and cell chemotaxis) were suppressed in enzalutamide-resistant prostate cancer cells. However, PD-L1 expression was highly up-regulated in these resistant cells. Moreover, the AR serves as a negative regulator of PD-L1 expression. Therefore, we suspected that AR might promote the formation of an immunosuppressive microenvironment in ARSI-resistant CRPC (Figure 1).
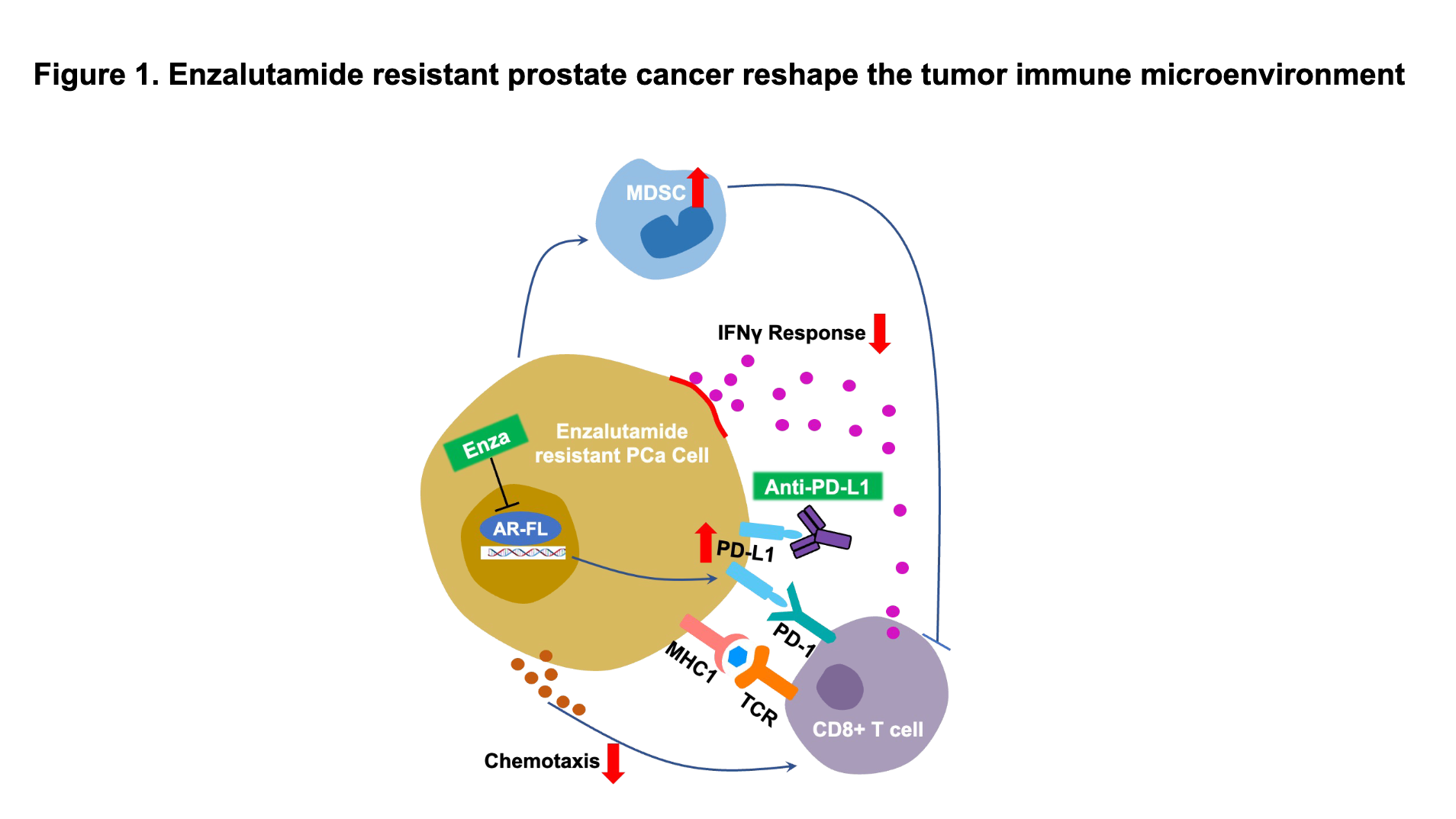
Enzalutamide treatment initially inhibited the growth of prostate tumors in mice; however, when mice developed enzalutamide resistance, MDSC infiltration and PD-L1 expression in the tumor tissues increased to form a highly immunosuppressive tumor microenvironment. Co-culturing bone marrow-derived cells with murine enzalutamide-resistant prostate cancer cells significantly increased the MDSC population, and enzalutamide treatment further increased the MDSC percentage.
In summary, immunosuppressive alterations in the tumor immune microenvironment can be promoted directly by enzalutamide-resistant CRPC cells, which promote self-immune evasion by inducing immunosuppressive cell infiltration and forming an immunosuppressive tumor microenvironment. A theoretical foundation was built for a deeper understanding of the mechanisms driving the formation of an immunosuppressive microenvironment and immune checkpoint inhibitor escape in enzalutamide-resistant CRPC.
Written by: Chengfei Liu, MD, PhD, Department of Urologic Surgery, UC Davis Comprehensive Cancer Center, University of California at Davis, Sacramento, CA, USA
Read the Abstract


