This review aims to serve as a valuable resource for nuclear medicine practitioners in PSMA-PET/CT reporting.
The review discusses the Prostate-Specific Membrane Antigen (PSMA) molecule and the impact of the different peptides on the biodistribution and diagnostic findings on PSMA-PET. It highlights specificity and positive predictive value as key diagnostic strengths of PSMA-PET, and the value of including preclinical risk in assigning significance of scan findings in report conclusions.1
The review gives an overview of the evidence for PSMA-PET in both PCa staging and biochemical recurrence (BCR). Most guidelines recommend PSMA-PET for staging intermediate- and high-risk patients being considered for definitive therapy and in BCR if PSA > 0.2-0.5 ng/mL.2,3 PSMA-PET is a cornerstone for assessment of patient eligibility for PSMA radioligand therapy (RLT). The review discusses the different criteria utilised to define eligibility and highlighted the role of whole body SUVmean as a positive predictor for patients’ outcomes with 177Lu-PSMA therapy. It also discusses the growing body of literature on the qualitative and quantitative criteria of post-therapy PSMA-PET and 177Lu-PSMA-SPECT in assessment of response to 177Lu-PSMA.
Standardized and synoptic reporting are also explained, particularly published frameworks for detailing PSMA-PET findings, with a specific focus on PROMISE version 2, PRIMARY, PSMA_RADS, and E-PSMA. An illustrative example of PSMA-PET in staging a patient with metastatic prostate cancer is provided with the synoptic report example incorporating the previous criteria (Figure 1 and 2).
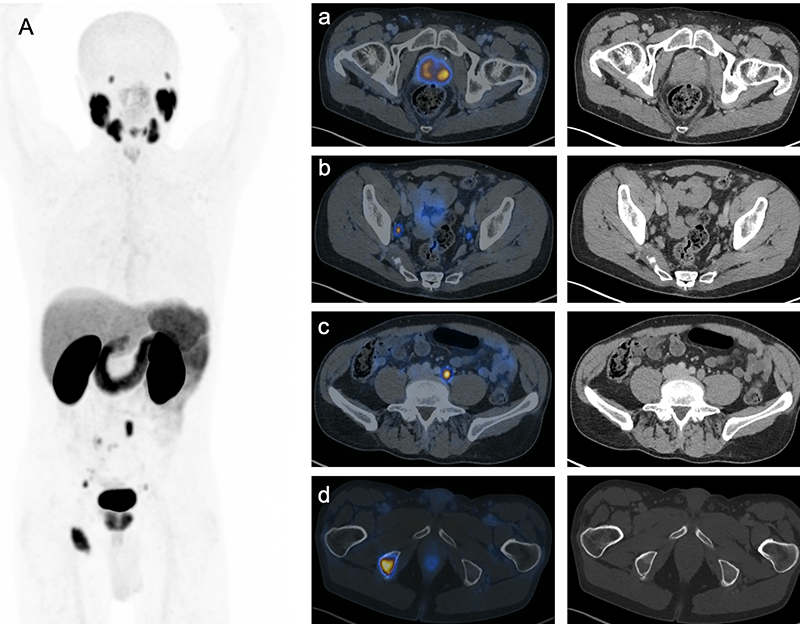
Figure 1. PSMA-PET/CT for initial staging of a 75-year-old man with metastatic prostate cancer. MIP (A), axial images (a- d) showing bilateral prostate lesions, right internal iliac and left common iliac nodal lesions and right ischial lytic lesion, respectively. Synoptic report is provided in Fig. 2.

Figure 2. Example of a synoptic report for the staging PSMA-PET/CT provided in Fig. 1.
Pitfalls in PSMA PET reporting are discussed with examples including recognizing benign PSMA-PET findings and refining acquisition protocols to bolster image diagnostic quality.
Written by: Mina Swiha MD, PhD1,2, Narjess Ayati MD1,3,4, Daniela Oprea Lager MD, PhD5, Francesco Ceci MD, PhD6,7, Louise Emmett MBChB, MD1,3,4
- Department of Theranostics and Nuclear Medicine, St Vincent’s Hospital, Sydney, Australia
- Nuclear Medicine Division, Department of Medical Imaging, University of Western Ontario, London, Canada
- St Vincent’s Clinical School, University of New South Wales, Sydney, Australia
- Garvan Institute of Medical Research, Sydney, Australia
- Department of Radiology & Nuclear Medicine, Amsterdam University Medical Centers, VU University. Medical Center, Cancer Center Amsterdam, De Boelelaan 1117, 1081 HV, Amsterdam, The Netherlands
- Division of Nuclear Medicine, IEO European Institute of Oncology IRCCS, Milan, Italy
- Department of Oncology and Hemato-Oncology, University of Milan, Italy
References:
- Yaxley JW, Raveenthiran S, Nouhaud FX, et al: Risk of metastatic disease on 68 gallium-prostate-specific membrane antigen positron emission tomography/computed tomography scan for primary staging of 1253 men at the diagnosis of prostate cancer [published correction appears in BJU Int. 2020 Mar;125(3):476]. BJU Int 124(3):401-407, 2019.
- Hofman MS, Lawrentschuk N, Francis RJ, et al: Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet 395(10231):1208-1216, 2020.
- Gillessen S, Bossi A, Davis ID, et al: Management of Patients with Advanced Prostate Cancer. Part I: Intermediate-/High-risk and Locally Advanced Disease, Biochemical Relapse, and Side Effects of Hormonal Treatment: Report of the Advanced Prostate Cancer Consensus Conference 2022. Eur Urol 83(3):267-293, 2023.


