The BOP trial was led by Prof Louise Emmett at St Vincent’s Hospital, Sydney and it assessed the safety and diagnostic performance of 64Cu SAR-Bombesin across two different groups of men:
- Participants with biochemical recurrence (BCR) of their prostate cancer who have negative PSMA positron emission tomography (PET) imaging scans or low PSMA expression disease.
- Participants with metastatic castrate resistant prostate cancer (mCRPC) who are not suitable for PSMA therapy.
Study results from the first cohort (BCR) have been presented at the European Association of Nuclear Medicine (EANM) 2023 Congress in Vienna, Austria, one of the most prestigious conferences in the nuclear medicine field.
Demographics showed that most participants in the BCR cohort of the BOP trial had undergone radical prostatectomy (96%), a surgery to remove the entire prostate gland and surrounding lymph nodes, with Prostate Specific Antigen (PSA) doubling time of 4.2 months (range 2.8 – 7.5; PSA mean 0.69 ng/ml, range 0.28 – 2.45) prior to entering the study. PSA is a blood test where high levels of PSA may indicate the presence of prostate cancer.
Participants in the BOP trial received the mean dose of 210 MBq of 64Cu SAR-Bombesin and imaged with Positron Emission Tomography (PET) at 1, 3 and 24 hours post-administration of the product. No adverse events from 64Cu-SAR-Bombesin administration were reported. 64Cu SAR-Bombesin PET avid disease was identified in over 30% of patients with negative or equivocal standard of care (SOC) PSMA PET (8/25, 32% detection rate).
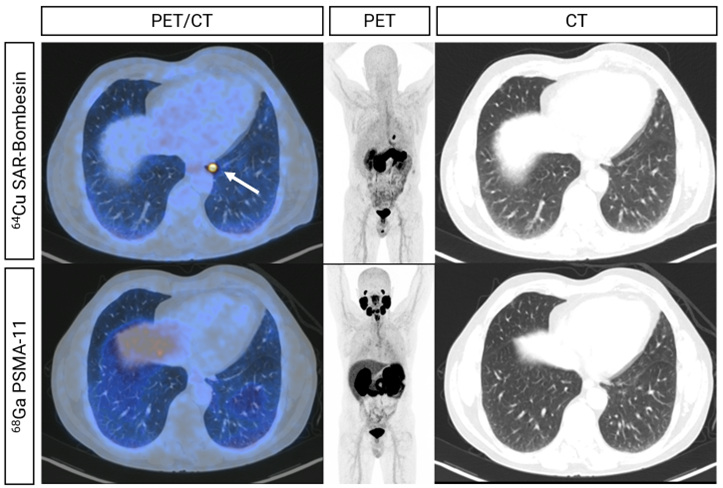
Figure 1. Fused, MIP (Maximum Intensity Projection) and CT (Computed Tomography) (left to right) images from 64Cu SAR-Bombesin (top) and PSMA (bottom) PET of a patient demonstrating a left subpleural lesion with 64Cu SAR-Bombesin uptake (arrow) without SOC PSMA uptake. This patient underwent a lobectomy with histopathology demonstrating metastatic prostate cancer.
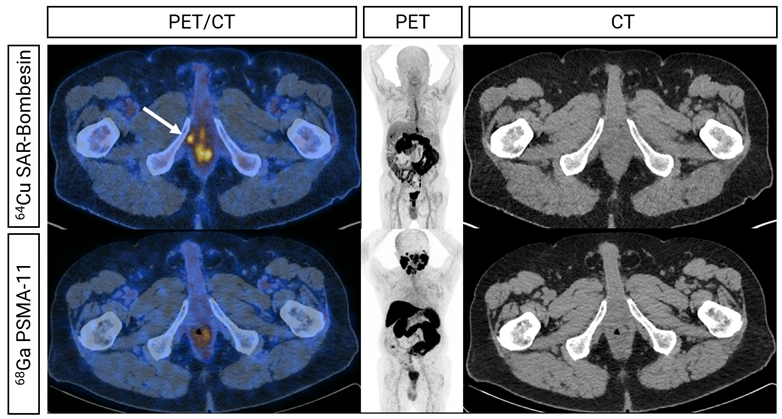
Figure 2– Fused, MIP and CT (left to right) images from 64Cu SAR-Bombesin (top) and PSMA (bottom) PET of a patient demonstrating uptake at the right urethral anastomosis on 64Cu SAR-Bombesin alone (arrow). This patient was managed with local radiotherapy with improvement in PSA post-treatment.
Clarity’s Executive Chairman, Dr Alan Taylor, commented, “What is most exciting about this data is the identification of lesions in the most difficult to treat patient group, who are negative on all other SOC imaging. This information arms clinicians with accurate diagnostic information and helps them determine the best course of treatment for their patients. In essence, for the patients that have had a positive SAR-Bombesin scan, this is the difference between having an incorrect negative cancer diagnosis leading to cancer progression and now having an effective treatment plan that may lead to long term remission. This patient-centric approach, reinforced by the flexibility in the timing of the scan, centralised product manufacture and its broad distribution to any imaging centre with a PET camera, would be a true paradigm shift in the management of prostate cancer. We are continuing to work diligently on the development of the SAR-Bombesin product in the US, including our Phase II SABRE trial (NCT05407311) as well as the theranostic Phase I/IIa COMBAT trial (NCT05633160), in order to make this product available to the patients who need it most and might not have any other diagnostic and therapeutic options available to them.”Prof Louise Emmett (St Vincent’s Hospital Sydney), Principal Investigator in the BOP trial, commented, “We are pleased with the results we have generated in the BOP trial, which have shown that 64Cu SAR-Bombesin can detect lesions in men with biochemically recurrent prostate cancer that are negative or equivocal on PSMA PET. We believe SAR-Bombesin has the potential to add value in biochemical recurrence of prostate cancer following definitive therapy. We look forward to continuing our collaboration with Clarity and also exploring the diagnostic and therapeutic benefits of SAR-Bombesin in not only prostate cancer which is PSMA negative, but also in other diseases such as breast cancer.”
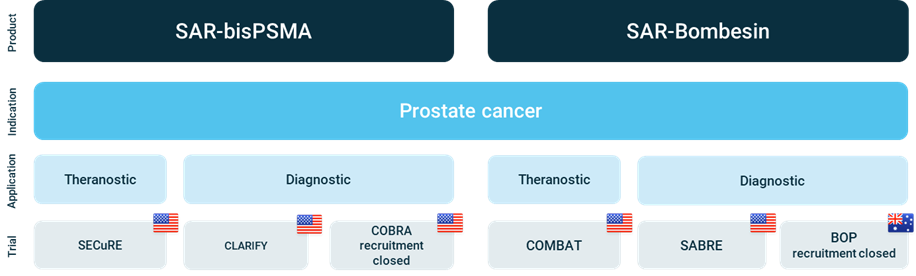
Overview of Clarity’s Prostate Cancer Clinical Trial Program
References:
- ClinicalTrials.gov Identifier: NCT05613842, https://clinicaltrials.gov/ct2/show/NCT05613842
Source: Clarity Pharmaceuticals. (2023). BOP Preliminary Trial Results Presented at EANM 2023 Conference [Press release]. https://www.claritypharmaceuticals.com/news/bopeanm2023/.


