After a great deal of vigorous debate, the group agreed to launch a multicenter randomized trial comparing MRI with targeted only biopsy to systematic biopsy in all. The rationale was that targeted only biopsy was the emerging strategy, and mandating systematic biopsies in the MRI group would muddy the waters.
The European trial was called the ‘PRECISION trial’, led by Drs. Veeru Kasivisvanathan and Carolyn Moore was an international collaboration. At the time, there were concerns that clinical equipoise had been lost in Europe. MRI was not available in Canada for men with elevated PSA, and we were confident that the trial would therefore accrue rapidly. Therefore we elected to run our own Canadian parallel trial. However, it took several years to obtain funding. PRECISION, despite concerns about equipoise, accrued very rapidly, and the Canadian trial, called PRECISE (to differentiate it from PRECISION), ultimately completed accrual about 2 years after the PRECISION trial.
PRECISE and PRECISION showed remarkably similar findings with respect to the primary endpoint (the proportion in each arm diagnosed with GG ≥ 2 PCa).1,2 Clinically significant prostate cancer was identified in PRECISE in 35% in the MRI arm vs 30% in the systematic arm, a 5% absolute difference, despite about 40% of men avoiding a biopsy. In PRECISION, the comparable figures for GG≥2 cancer were 38% and 26%, a 12% absolute difference. GG1 PCa diagnosis was reduced in PRECISE by 12% (22% to 10%) and by 13% in PRECISION.
These trials were designed to be combined in a meta-analysis to enhance the power of the individual trials.3
An unanswered question in both studies, inherent to the study design, was the outcome in the 40% of patients in the MRI arm who avoided a biopsy, as well as the outcome in the patients in both groups with a negative biopsy or who were diagnosed with GG1 disease and managed with surveillance. In the PRECISE trial, we built in 2 years of follow-up, and a mandated 2 year MRI in all patients still on trial. The monograph recently published presents these results. In fact, we have extended the planned follow-up to a total of 8 years. This should provide us with important data on the likelihood, with long-term follow-up, of late diagnosis of significant cancer in the unbiopsied MRI negative group.
The data at 2 years is reassuring. At 2 years, with an MRI and targeted biopsy in both groups, there was no difference between the groups in the subsequent diagnosis of GG ≥ 2 cancer. In other words, despite more than a one third reduction in the number of biopsies, and a median of 3 vs 12 cores in those who did have a biopsy, the MRI group did not harbor a larger reservoir of undiagnosed significant cancers compared to the systematic biopsy group. Further, none of the MRI patients upgraded at 2 years had GG ≥ 3. At 2 years, 8% of the systematic biopsy group and 5% of the MRI group were found to have GG ≥ 2 cancer; and 3% and 0% respectively, were found to have GG 3. This data reinforces the safety of a targeted biopsy only strategy for prostate cancer diagnosis.
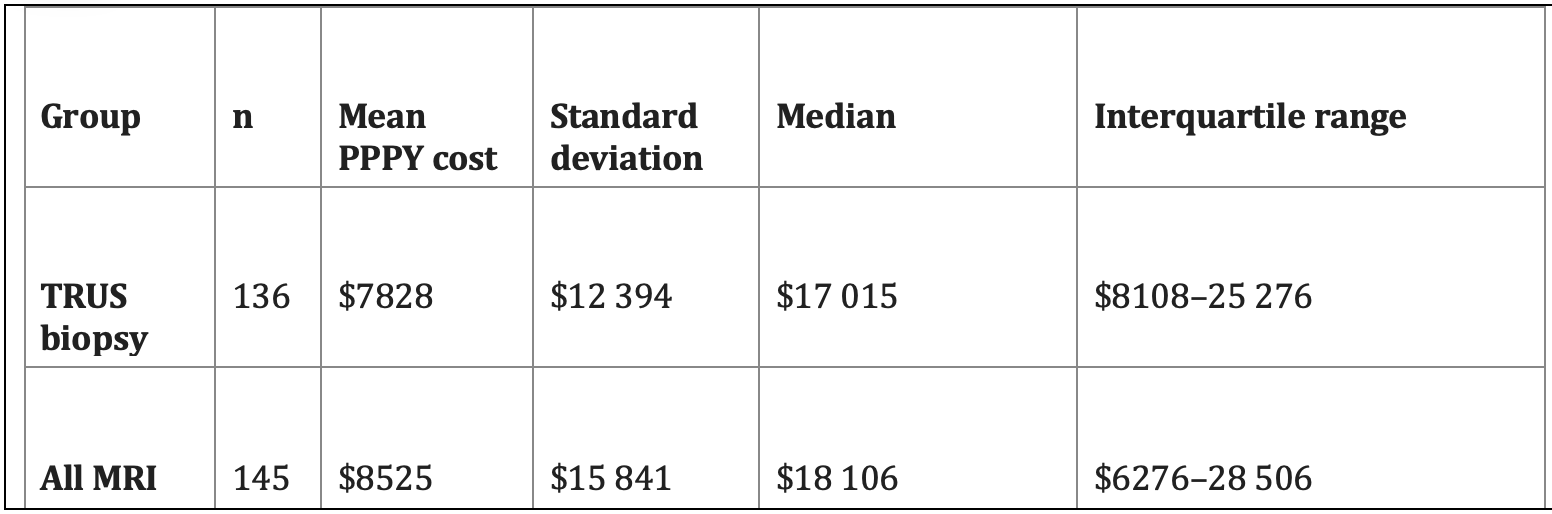
We have also performed an economic analysis of the patients in the PRECISE study, based on linking the Ontario based patients (281 of the 453 patients in the study) to provincial insurance claims.4 This analysis showed that the systematic biopsy group had considerably greater rates of post biopsy hospitalization for sepsis or hemorrhage. This reflects the avoidance of biopsy in 38%, and 12 cores vs a median of 3 cores in the MRI group. (Almost all patients had transrectal biopsies). The mean per person per year (PPPY) costs for the systematic biopsy and MRI groups (including the cost of the MRI) were $7828 and $8525, respectively (Table 1). This analysis did not consider the additional costs of managing the increased numbers of active surveillance patients in the systematic biopsy arm. This suggests that, compared to systematic transrectal biopsies, MRI with targeted only biopsy is relatively cost-effective, despite the cost of MRI. (Whether this will be true with the shift to trans-perineal biopsy is unclear).
Table 1. Ontario Health Insurance claims in the 12 months following study entry (including MRI costs)
The PRECISE trial has been impactful. Cancer Care Ontario, whose guidelines tend to be adopted nationally in Canada, changed their prostate cancer guideline to recommend MRI prior to biopsy about 2 weeks after the initial publication of the PRECISE trial. The data in the 2 year follow-up study reinforces the safety of this strategy.
Long term follow-up (to 8 years) of the PRECISE cohort is ongoing and will be the subject of subsequent reports.
A remaining clinical question is the role of systematic biopsies in men having a targeted biopsy. Recent genomic and clinical data suggest strongly that MRI invisible cancers have favorable genetic characteristics and indolent natural history, implying that systematic biopsies to detect invisible cancers are not necessary. An additional question is whether high resolution micro-ultrasound, which has several advantages over MRI in terms of cost, absence of contrast requirement, and patient convenience, can achieve similar results. A large randomized trial comparing MRI to high resolution micro-ultrasound is currently in progress.5
In conclusion, the evidence from PRECISION and PRECISE provides robust level 1 evidence that MRI with targeted only biopsies is superior to systematic biopsies in all patients. The 2-year follow-up data presented in this article reinforces the safety of this approach. These studies have deservedly changed practice throughout the world.
Written by: Laurence Klotz, CM, MD, FRCSC, Sunnybrook Chair of Prostate Cancer Research, University of Toronto
References:
- Klotz L, Chin J, Black PC, Finelli A, Anidjar M, Bladou F, Mercado A, Levental M, Ghai S, Chang SD, Milot L, Patel C, Kassam Z, Moore C, Kasivisvanathan V, Loblaw A, Kebabdjian M, Earle CC, Pond GR, Haider MA, Comparison of Multiparametric Magnetic Resonance Imaging-Targeted Biopsy With Systematic Transrectal Ultrasonography Biopsy for Biopsy-Naive Men at Risk for Prostate Cancer: A Phase 3 Randomized Clinical Trial.JAMA Oncol. 2021 Apr 1;7(4):534-542
- Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, Briganti A, Budäus L, Hellawell G, Hindley RG, Roobol MJ, Eggener S, Ghei M, Villers A, Bladou F, Villeirs GM, Virdi J, Boxler S, Robert G, Singh PB, Venderink W, Hadaschik BA, Ruffion A, Hu JC, Margolis D, Crouzet S, Klotz L, Taneja SS, Pinto P, Gill I, Allen C, Giganti F, Freeman A, Morris S, Punwani S, Williams NR, Brew-Graves C, Deeks J, Takwoingi Y, Emberton M, Moore CM; PRECISION Study Group Collaborators MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. .N Engl J Med. 2018 May 10;378(19):1767-1777.
- Kasivisvanathan V, Chan VW, Clement KD, Levis B, Haider M, Agarwal R, Emberton M, Pond GR, Takwoingi Y, Klotz L, Moore CM; VISION study collaborators. PLoS One A protocol for the VISION study: An indiVidual patient data meta-analysis of randomised trials comparing MRI-targeted biopsy to standard transrectal ultraSound guided bIopsy in the detection of prOstate cancer. . 2022 Feb 3;17(2):e0263345
- Seung SJ, Saherawala H, Nguyen L, Gatley JM, Liu N, Kebabdjian M, Earle C, Klotz L, Mittmann N Hospital encounters and associated costs of prostate evaluation for clinically important disease MRI vs. standard evaluation procedures (PRECISE) study from a provincial-payer perspective.Can Urol Assoc J. 2023 Aug;17(8):280-284
- Klotz L, Andriole G, Cash H, Cooperberg M, Crawford ED, Emberton M, Gomez-Sancha F, Klein E, Lughezzani G, Marks L, Montorsi F, Salomon G, Sanchez-Salas R, Shore N, Taneja S Optimization of prostate biopsy - Micro-Ultrasound versus MRI (OPTIMUM): A 3-arm randomized controlled trial evaluating the role of 29 MHz micro-ultrasound in guiding prostate biopsy in men with clinical suspicion of prostate cancer.Contemp Clin Trials. 2022 Jan;112:106618


