CLARIFY derives from “Positron Emission Tomography using 64Cu-SAR-bisPSMA in participants with high-risk prostate cancer prior to radical prostatectomy: A prospective, single-arm, multi-centre, blinded-review, Phase III diagnostic performance study”. It is a non-randomised, open-label clinical trial in 383 participants.
The aim of the Phase III trial is to assess the diagnostic performance of 64Cu-SAR-bisPSMA PET to detect prostate cancer within lymph nodes located in the pelvic region. Evaluation will take place over 2 imaging timepoints, Day 1 (day of administration) and Day 2 (approximately 24 hours post administration). CLARIFY is expected to begin recruitment in late 2023.
Clarity’s Executive Chairman, Dr Alan Taylor, commented, “We are very excited to move one step closer to initiating our first registrational Phase III trial. With recent positive and valuable guidance from the US FDA in relation to our 64Cu-SAR-bisPSMA program, we look forward to commencing recruitment into the CLARIFY trial shortly and to gathering more data on this next-generation product to confirm the compelling preclinical and clinical trial results to date.
“The positive results from our completed PROPELLER2 trial showed that 64Cu-SAR-bisPSMA is safe and its uptake in PSMA-expressing cancer lesions was significantly higher compared to an approved standard-of-care PSMA imaging agent for prostate cancer in Australia and the US. This may enable diagnosis of additional and smaller lesions, which we observed in our PROPELLER2 trial, and we are eager to investigate the further benefits of delayed imaging, particularly in this patient population, a characteristic not available to the first generation of PSMA diagnostic agents. Furthermore, we believe that the additional shelf-life of up to 48 hours will not only allow clinics greater flexibility in scheduling of the scans, but also improve patients’ access to care in clinics and geographic areas where the short half-life of current PSMA PET tracers restricts the use of radiopharmaceuticals.”PSI’s Senior Director of Operations, Rhonda Critchlow, commented, “Using our global database of over 400 radiopharmaceutical sites, we will be able to identify sites with the best resources and capabilities for the CLARIFY trial. We are excited to begin our collaboration with Clarity and will focus on the startup of high-performing sites to achieve the first patient, in the shortest time possible. We believe that a myriad of clinical, logistical and manufacturing benefits of Clarity’s Targeted Copper Theranostics platform holds promise of improving treatment outcomes for patients with cancer and look forward to working together on achieving this important goal.”
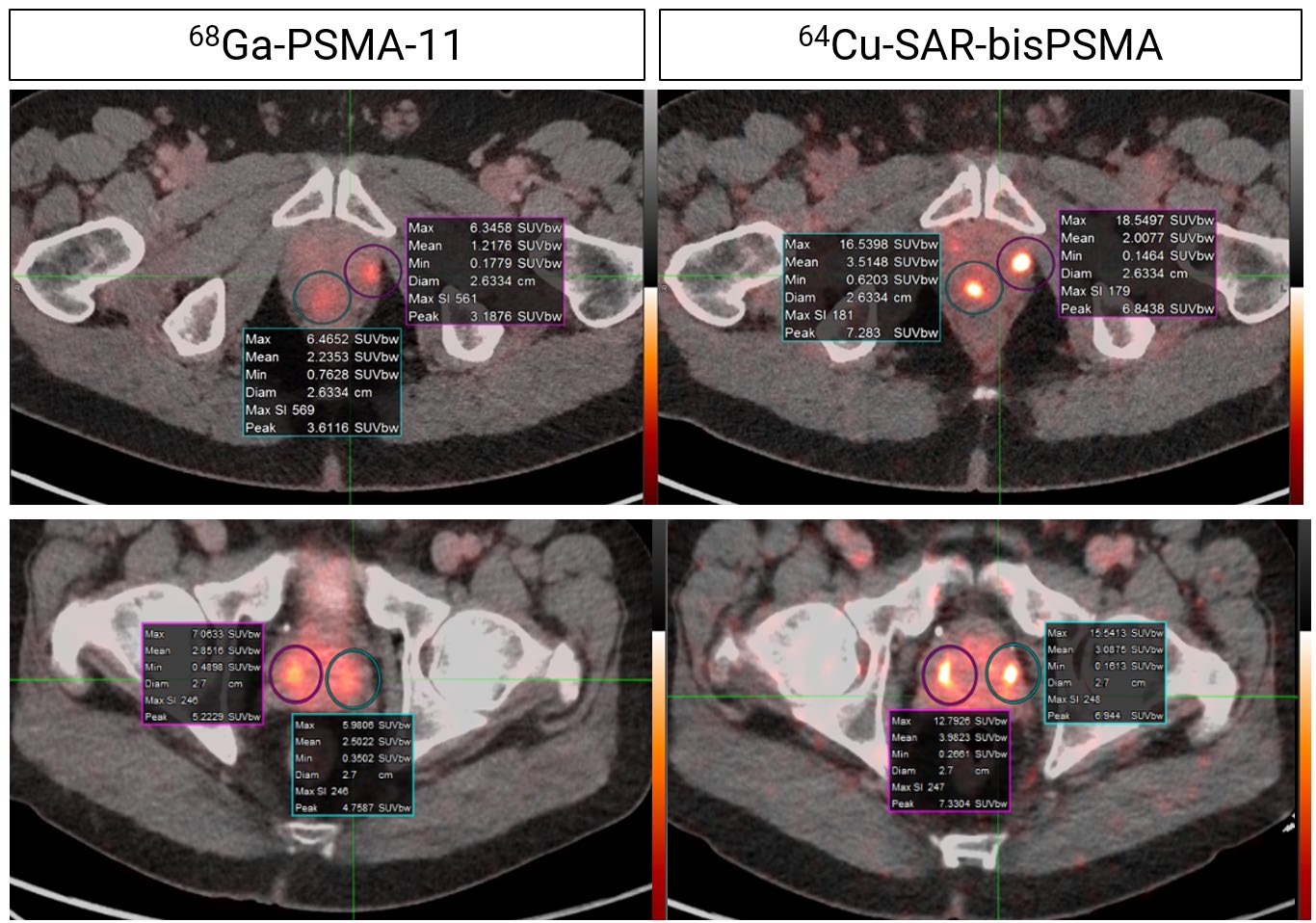
Concordant lesions on 64Cu-SAR-bisPSMA (200 MBq) and 68Ga-PSMA-11 PET/CT consistently showed higher SUVmax, SUVmean and tumor to background ratio with 64Cu-SAR-bisPSMA compared to 68Ga-PSMA-11. SUV: standardised uptake value. PROPELLER study.
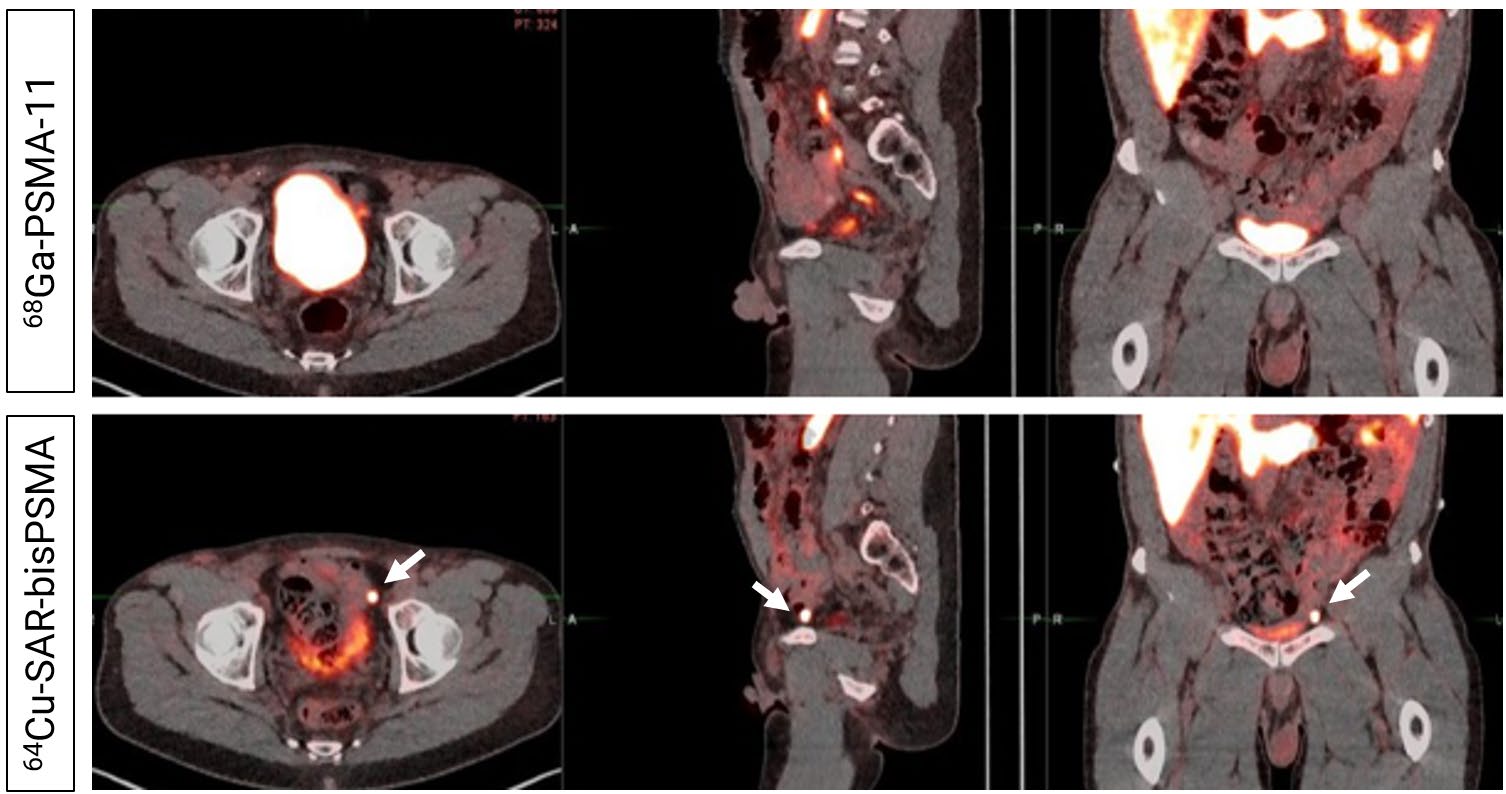
Readers did not detect uptake in pelvic lymph nodes on the 68Ga-PSMA-11 PET/CT (Top). PET/CT demonstrated uptake of 64Cu-SAR-bisPSMA (Bottom) in a left pelvic lymph node according to both Readers. Prostate cancer was confirmed via histopathology. Arrows highlight the node detected on 64Cu-SAR-bisPSMA PET/CT. PROPELLER study.
References:
- Positron Emission Tomography Using 64Cu-SAR-bisPSMA in Participants With High-risk Prostate Cancer Prior to Radical Prostatectomy: A Prospective, Single-arm, Multi-center, Blinded-review, Phase 3 Diagnostic Performance Study – CLARIFY. ClinicalTrials.gov ID NCT06056830.
- Lengyelova E, Wong V, Lenzo N, Parker M, Emmett L. 64Cu-SAR-bisPSMA (PROPELLER) positron emission tomography (PET) imaging in patients with confirmed prostate cancer. ASCO 2023. Poster available at: claritypharmaceuticals.com/pipeline/scientific_presentations
Source: Clarity Pharmaceuticals. 2023. Clarity and PSI kick off SAR-bisPSMA Phase III [Press release]. https://www.claritypharmaceuticals.com/news/psi/.


