Reno, Nevada (UroToday.com) -- December 18, 2023 – Lantheus Holdings, Inc. (Lantheus) (NASDAQ: LNTH) and POINT Biopharma Global Inc. (POINT) (NASDAQ: PNT) announced statistically significant topline results from the pivotal Phase 3 SPLASH study evaluating the efficacy and safety of 177Lu-PNT2002, a prostate-specific membrane antigen (PSMA)-targeted radioligand therapy (RLT), in patients with metastatic castration-resistant prostate cancer (mCRPC) after progression on an androgen receptor pathway inhibitor (ARPI).
“There is an urgent unmet need for targeted treatment options for mCRPC patients, particularly for those whose cancer has progressed on androgen receptor pathway inhibitors,” said Dr. Kim Chi, SPLASH Principal Investigator, Medical Oncologist, BC Cancer. “The SPLASH study results demonstrate that 177Lu-PNT2002 is well-tolerated and has the potential to play an important role in addressing those needs for patients with chemotherapy-naïve mCRPC.”The SPLASH trial met its primary endpoint, demonstrating a median radiographic progression-free survival (rPFS) per blinded independent central review of 9.5 months for patients treated with 177Lu-PNT2002, compared to 6.0 months for patients treated with ARPI in the control arm, a statistically significant 29% reduction in the risk of radiographic progression or death (hazard ratio [HR] 0.71; p=0.0088). At the time of the analysis, interim overall survival (OS) results were immature (46% of protocol-specified target OS events reached), the HR was 1.11. The companies expect additional, follow-up data in 2024 prior to the potential submission of a New Drug Application (NDA).
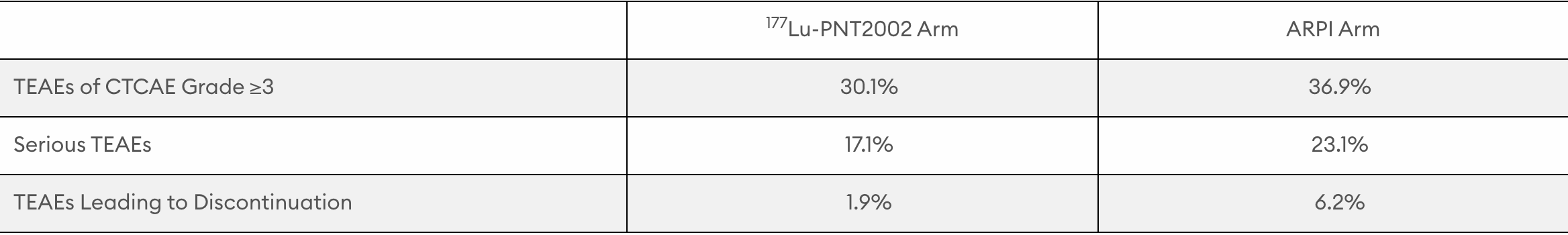
177Lu-PNT2002 demonstrated a favorable safety profile with grade ≥3 treatment-emergent adverse events (TEAEs) per Common Terminology Criteria for Adverse Events (CTCAE), serious TEAEs, and TEAEs leading to discontinuation occurring at lower rates in the 177Lu-PNT2002 arm than in the ARPI arm (30.1%, 17.1%, and 1.9% vs. 36.9%, 23.1%, and 6.2%, respectively).
The open-label study randomized 412 patients with PSMA-expressing mCRPC who had progressed on ARPI therapy and either refused or were not eligible for chemotherapy, in a 2:1 randomization ratio (177Lu-PNT2002: control group). At the time of the analysis, 84.6% of patients who experienced progressive disease in the control arm subsequently crossed over to receive 177Lu-PNT2002. SPLASH was conducted across the United States, Canada, Europe, and the United Kingdom. Eighty percent of SPLASH patients resided in North America and approximately 10% of all participants were Black or African American.
"The success of 177Lu-PNT2002 in this trial demonstrates the value of treating patients with radioligand therapy at this stage of the disease continuum. With only four treatment administrations over a 32-week period, this regimen provides reduced treatment intensity compared to the control arm, while also delaying disease progression with lower toxicity,” said Neil Fleshner, M.D., Co-founder and Chief Medical Officer at POINT Biopharma. “We extend our deepest gratitude to the SPLASH study participants and their families and caregivers, as well as the investigators and their hard-working staff."“We are encouraged by the results of the SPLASH trial, which showed the benefits that 177Lu-PNT2002 offers to patients with mCRPC. We are proud to be at the forefront of advancing the potential of targeted RLT with 177Lu-PNT2002. As the leading radiopharmaceutical-focused company, we are committed to providing clinicians with cutting-edge options to fight disease and improve patient outcomes,” said Jean-Claude Provost, M.D., Chief Medical Officer at Lantheus. “We look forward to sharing additional data in the future, and to collaborating with regulatory authorities and POINT Biopharma to bring this promising therapy to the prostate cancer patient community.”
Full SPLASH trial results will be presented at a future medical congress.
Source: Lantheus Holdings, Inc. (2023). Lantheus and POINT Biopharma Announce Positive Topline Results from Pivotal SPLASH Trial in Metastatic Castration-Resistant Prostate Cancer [Press Release]. https://lantheusholdings.gcs-web.com/news-releases/news-release-details/lantheus-and-point-biopharma-announce-positive-topline-results.


