In the phase 3 HERO study, relugolix, an oral, highly selective receptor GnRH antagonist, demonstrated sustained and superior suppression of testosterone to castration levels, as well as a 54% reduction in the risk of MACE, relative to the LHRH agonist leuprolide acetate (leuprolide).8 Of note, over 90% of patients in the HERO trial had at least one CV risk factor, i.e., lifestyle risk factors, CV or cerebrovascular risk factors, or a history of MACE. In this study, we evaluated the impact of concomitant CV therapies on efficacy and safety in the HERO study.9
In this post-hoc subgroup analysis of the HERO study, we compared the primary endpoint (sustained testosterone suppression to castrate levels [<50 ng/dL] from day 29 through 48 weeks) and adverse events (AEs) in patients treated with relugolix or leuprolide who concomitantly used CV medications.
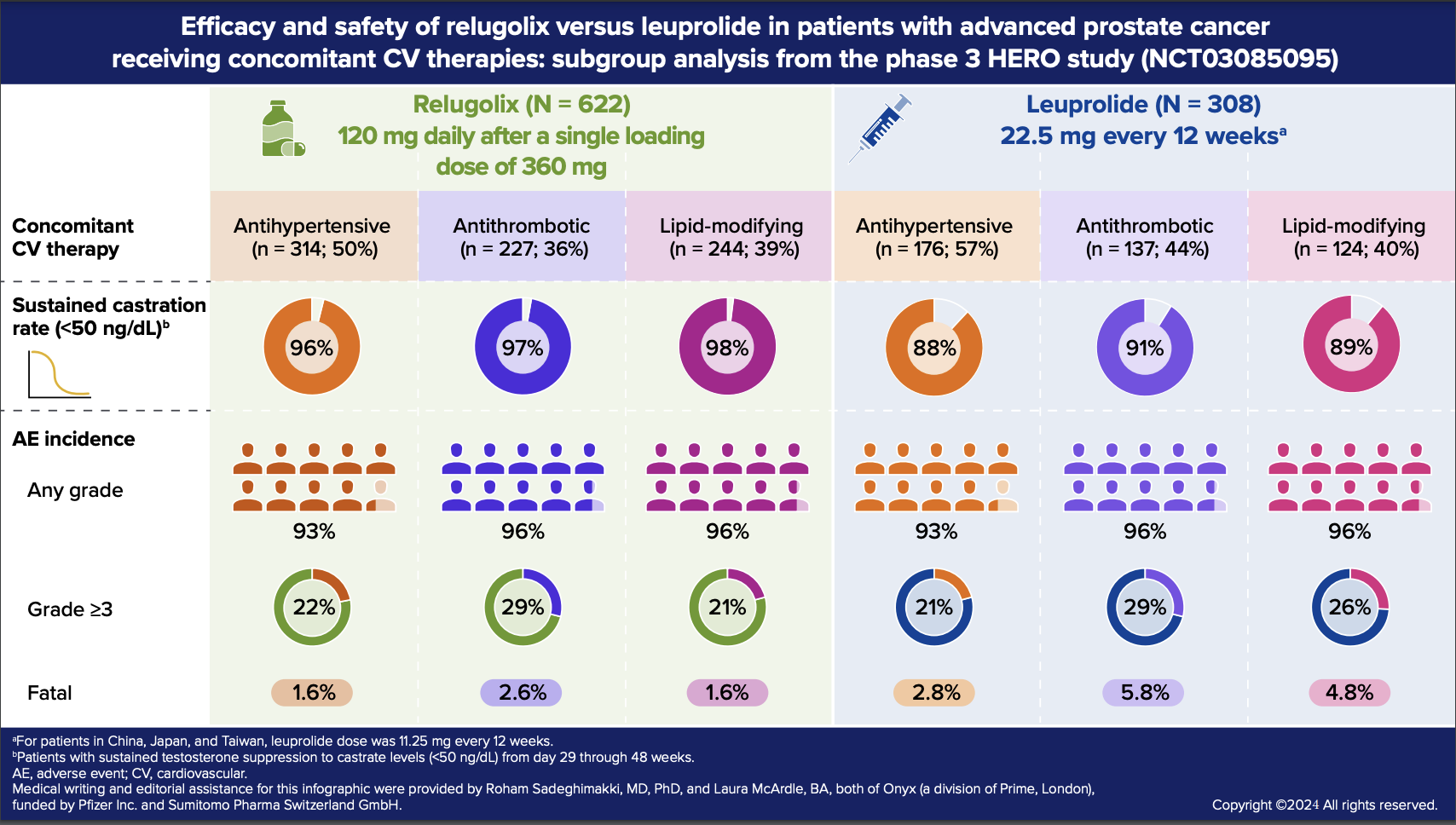
Among patients in the relugolix cohort (n=622) and leuprolide cohort (n=308), 73.3% and 81.8% received at least one concomitant CV medication, respectively. The estimated rates of sustained castration for the subgroups of patients receiving antihypertensives, antithrombotics, and lipid-modifying agents were generally comparable to those in the overall HERO population.
Consistent with the overall HERO population, hot flash was the most common AE across all three CV medication subgroups in both treatment cohorts (relugolix ~58%; leuprolide 49‒59%), followed by fatigue, constipation, diarrhea, arthralgia, and hypertension, each with >10% incidence in at least one subgroup. In the relugolix cohort, the proportion of patients with concomitant CV medication use who experienced an AE leading to treatment withdrawal or interruption was similar or slightly higher relative to the overall population that received relugolix in the HERO study. In the leuprolide cohort, none of the patients who received CV medications and <1% of the overall population experienced AEs leading to treatment withdrawal or interruption.
Some notable differences in AE patterns were observed between the overall HERO population and this subgroup analysis. In both treatment cohorts, grade ≥3 AEs were proportionally higher in the subgroups receiving CV medications compared with the overall population, particularly in the subgroup receiving antithrombotics. Similarly, in both treatment cohorts, fatal AEs occurred more frequently in the subgroups of patients concomitantly treated with antithrombotics or lipid-modifying agents relative to the overall population, with fewer fatal AEs (including CV-related fatal events) in the relugolix cohort than in the leuprolide cohort.
In our subgroup analysis, relugolix suppressed testosterone with efficacy consistent with that of the overall HERO population and was generally well-tolerated when given with concomitant CV therapies. These findings support the use of relugolix as an ADT option in patients with advanced prostate cancer who receive concomitant CV-related medications due to increased risk of CV events. However, certain subgroups of patients when treated with relugolix or leuprolide and CV drugs are at a high risk of serious treatment-related AEs.
Written by: Neal D. Shore, MD, FACS, Carolina Urologic Research Center/GenesisCare US, Myrtle Beach, SC, USA
References:
- Sturgeon KM, Deng L, Bluethmann SM, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40(48):3889-3897.
- Chan JSK, Satti DI, Lee YHA, et al. Temporal trends in cardiovascular burden among patients with prostate cancer receiving androgen deprivation therapy: a population-based cohort study. Br J Cancer. 2023;128(12):2253-2260.
- Kakarla M, Ausaja Gambo M, Yousri Salama M, et al. Cardiovascular effects of androgen deprivation therapy in prostate cancer patients: a systematic review. Cureus. 2022;14(6):e26209.
- Tsai HK, D'Amico AV, Sadetsky N, Chen MH, Carroll PR. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007;99(20):1516-1524.
- Hu JR, Duncan MS, Morgans AK, et al. Cardiovascular effects of androgen deprivation therapy in prostate cancer: Contemporary meta-analyses. Arterioscler Thromb Vasc Biol. 2020;40(3):e55-e64.
- Nelson Adam J, Lopes Renato D, Hong H, et al. Cardiovascular effects of GnRH antagonists compared with agonists in prostate cancer. JACC: CardioOncology. 2023;5(5):613-624.
- Gu L, Li X, Liu W. Adverse cardiovascular effect following gonadotropin-releasing hormone antagonist versus GnRH agonist for prostate cancer treatment: A systematic review and meta-analysis. Front Endocrinol (Lausanne). 2023;14:1157857.
- Shore ND, Saad F, Cookson MS, et al. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. 2020;382(23):2187–2196.
- Shore ND, Mehlhaff BA, Cookson MS, et al. Impact of concomitant cardiovascular therapies on efficacy and safety of relugolix vs leuprolide: Subgroup analysis from HERO study in advanced prostate cancer. Adv Ther. 2023;40(11):4919-4927.
Dr. Neal D. Shore reports consulting or advisory roles with or research funding from AbbVie, Amgen, Astellas Pharma Inc., AstraZeneca, Bayer, Bristol Myers Squibb, Dendreon, Exact Sciences, Ferring, FerGene, Janssen, MDx Health, Merck, Nymox, Pfizer Inc., Sanofi, Sumitomo Pharma Switzerland GmbH (formerly Myovant Sciences), and Tolmar.
Medical writing and editorial assistance were provided by Roham Sadeghimakki, MD, PhD, and Laura McArdle, BA, both of Onyx (a division of Prime, London, UK), funded by Pfizer Inc. and in collaboration with Sumitomo Pharma Switzerland GmbH. The HERO study was sponsored by Sumitomo Pharma Switzerland GmbH.
Read the Abstract


