Various methods developed and tested in clinical trials aim to target cancer cells expressing PSMA, showing promising outcomes, with the effectiveness of PSMA-targeted therapies heavily reliant on the level of PSMA expressed on the cell surface. Yet, PSMA heterogeneity across multiple levels (interpatient, intertumoral, and intratumoral) has been documented, contributing to the development of resistance against PSMA-targeted therapies.2-4
Understanding the molecular mechanisms governing PSMA regulation is crucial for designing strategies to enhance its expression. HOXB13 and AR are identified as pivotal transcription factors influencing PSMA expression positively and negatively, respectively 3. Moreover, recent findings highlight the role of epigenetic regulation in controlling FOLH1 transcription.2,3 Yet, the complete understanding of how PSMA expression is regulated remains unclear. Within the review, we utilized data from our recently developed Prostate Cancer Transcriptome Atlas5 to compile a roster of transcriptional regulators highly associated with PSMA. This list may offer potential candidate genes involved in its regulation.
In addition, in the review, we rationalize combination therapies, as several established therapeutic approaches not only have the potential to increase PSMA levels but also to enhance DNA damage caused by existing radioligands (Figure 1).
Therapeutic modalities such as AR inhibition not only can increase PSMA levels but also impair DNA repair mechanisms, potentially improving the effectiveness of PSMA-targeted radionuclide therapy (RLT).6,7 The PSMAddition trial in metastatic hormone-sensitive prostate cancer and the ENZA-p trial in castration resistant prostate cancer are ongoing to explore this combination therapy.8,9
Chemotherapy remains a primary treatment option for prostate cancer and neuroendocrine prostate cancer (NEPC) patients. Interestingly, certain chemotherapeutic drugs that induce DNA damage have been found to upregulate PSMA protein levels, suggesting a possibility to enhance PSMA-targeted therapy outcomes through chemotherapy-induced PSMA upregulation.10
Poly (ADP-ribose) polymerase inhibitors (PARPi) have shown effectiveness in inducing synthetic lethality in prostate cancer cells with DNA repair abnormalities. Combining PARPi with PSMA-targeted RLT might amplify the therapeutic effects, as PARPi are crucial for repairing single-strand DNA breaks induced by beta minus particle emitters used in RLT.11 Currently, the LuPARP trial is investigating this combinatorial strategy.12
Immune checkpoint inhibitors have shown efficacy in a subset of prostate cancer patients with high mutational burden and microsatellite instability (MSI). Combining these inhibitors with PSMA-targeted RLT may enhance immune responses through the radiation-induced neoantigens formation on cancer cells, potentially broadening the application of immunotherapy in prostate cancer treatment.13 Multiple ongoing trials are assessing the combination of immune checkpoint inhibitors with PSMA-RLT.14-16
The observed increase in PSMA expression following DNA double-strand breaks suggests the possibility of synergizing PSMA-targeted RLT with itself. A dosing regimen involving sequential PSMA-RLT injections exploiting the upregulation induced by the initial dose is a potential strategy.
In conclusion, a better understanding of PSMA expression is mandatory to fight intra- and intertumoral PSMA heterogeneity as the identification of new regulators of PSMA could reveal novel ways to increase PSMA expression, for example by activating or inhibiting a positive or a negative PSMA regulator, respectively, and augment PSMA-targeted therapies. In addition, this will provide the basis for synergistic combination therapies.
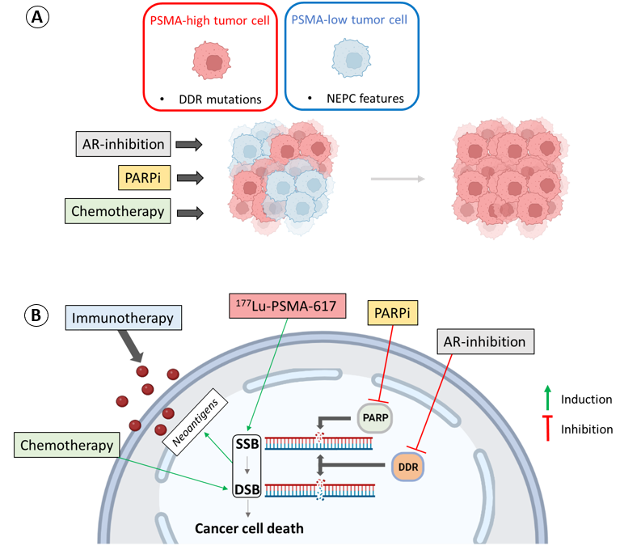
Figure 1. Synergism of different therapeutic approaches with PSMA-targeted therapies.
- Institute of Oncology Research, Bellinzona, Switzerland; Faculty of Biomedical Sciences, Università della Svizzera italiana, Lugano, Switzerland.
- Department of Chemistry and Applied Biosciences, ETH Zurich, Zurich, Switzerland; Center for Radiopharmaceutical Sciences ETH-PSI, Paul Scherrer Institute, Villigen-PSI, Switzerland.
- Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
- The Institute of Cancer Research, London, UK; The Royal Marsden Hospital, London, UK.
- Institute of Oncology Research, Bellinzona, Switzerland; Faculty of Biomedical Sciences, Università della Svizzera italiana, Lugano, Switzerland.
- Wright GL, Jr., Haley C, Beckett ML, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol. Jan-Feb 1995;1(1):18-28. doi:10.1016/1078-1439(95)00002-y
- Sayar E, Patel RA, Coleman IM, et al. Reversible epigenetic alterations mediate PSMA expression heterogeneity in advanced metastatic prostate cancer. JCI Insight. Apr 10 2023;8(7)
- Bakht MK, Yamada Y, Ku SY, et al. Landscape of prostate-specific membrane antigen heterogeneity and regulation in AR-positive and AR-negative metastatic prostate cancer. Nat Cancer. Apr 10 2023;doi:10.1038/s43018-023-00539-6
- Mannweiler S, Amersdorfer P, Trajanoski S, Terrett JA, King D, Mehes G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol Oncol Res. Jun 2009;15(2):167-72. doi:10.1007/s12253-008-9104-2
- Bolis M, Bossi D, Vallerga A, et al. Dynamic prostate cancer transcriptome analysis delineates the trajectory to disease progression. Nat Commun. Dec 2 2021;12(1):7033. doi:10.1038/s41467-021-26840-5
- Wright GL, Jr., Grob BM, Haley C, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. Aug 1996;48(2):326-34. doi:10.1016/s0090-4295(96)00184-7
- Polkinghorn WR, Parker JS, Lee MX, et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. Nov 2013;3(11):1245-53. doi:10.1158/2159-8290.CD-13-0172
- Sartor AO, Tagawa ST, Saad F, et al. PSMAddition: A phase 3 trial to compare treatment with 177Lu-PSMA-617 plus standard of care (SOC) versus SOC alone in patients with metastatic hormone-sensitive prostate cancer. Journal of Clinical Oncology. 2022;40(6_suppl):TPS210-TPS210. doi:10.1200/JCO.2022.40.6_suppl.TPS210
- Emmett L, Subramaniam S, Zhang AY, et al. ENZA-p: A randomized phase II trial using PSMA as a therapeutic agent and prognostic indicator in men with metastatic castration-resistant prostate cancer treated with enzalutamide (ANZUP 1901). Journal of Clinical Oncology. 2021;39(6_suppl):TPS177-TPS177. doi:10.1200/JCO.2021.39.6_suppl.TPS177
- Sheehan B, Neeb A, Buroni L, et al. Prostate-Specific Membrane Antigen Expression and Response to DNA Damaging Agents in Prostate Cancer. Clin Cancer Res. Jul 15 2022;28(14):3104-3115. doi:10.1158/1078-0432.CCR-21-4531
- Nonnekens J, van Kranenburg M, Beerens CE, et al. Potentiation of Peptide Receptor Radionuclide Therapy by the PARP Inhibitor Olaparib. Theranostics. 2016;6(11):1821-32. doi:10.7150/thno.15311
- Sandhu S, Joshua AM, Emmett L, et al. LuPARP: Phase 1 trial of 177Lu-PSMA-617 and olaparib in patients with metastatic castration resistant prostate cancer (mCRPC). Journal of Clinical Oncology. 2023;41(16_suppl):5005-5005. doi:10.1200/JCO.2023.41.16_suppl.5005
- Patel RB, Hernandez R, Carlson P, et al. Low-dose targeted radionuclide therapy renders immunologically cold tumors responsive to immune checkpoint blockade. Sci Transl Med. Jul 14 2021;13(602)doi:10.1126/scitranslmed.abb3631
- Sandhu S, Joshua AM, Emmett L, et al. PRINCE: Phase I trial of 177Lu-PSMA-617 in combination with pembrolizumab in patients with metastatic castration-resistant prostate cancer (mCRPC). Journal of Clinical Oncology. 2022;40(16_suppl):5017-5017. doi:10.1200/JCO.2022.40.16_suppl.5017
- Aggarwal RR, Sam SL, Koshkin VS, et al. Immunogenic priming with 177Lu-PSMA-617 plus pembrolizumab in metastatic castration resistant prostate cancer (mCRPC): A phase 1b study. Journal of Clinical Oncology. 2021;39(15_suppl):5053-5053. doi:10.1200/JCO.2021.39.15_suppl.5053
- Sandhu S, Subramaniam S, Hofman MS, et al. Evolution: Phase II study of radionuclide 177Lu-PSMA-617 therapy versus 177Lu-PSMA-617 in combination with ipilimumab and nivolumab for men with metastatic castration-resistant prostate cancer (mCRPC; ANZUP 2001). Journal of Clinical Oncology. 2023;41(6_suppl):TPS271-TPS271. doi:10.1200/JCO.2023.41.6_suppl.TPS271


