We previously assessed the learning curve for biochemical recurrence after robot-assisted radical prostatectomy, showing that outcomes of less experienced surgeons did not differ from those of more experienced ones.2 This finding differed from similar analyses performed in patients receiving open or laparoscopic radical prostatectomy, suggesting that there might be differences in populations and in how a certain operation is learned and performed across different surgical approaches, or a combination of these factors.3,4
In patients undergoing radical surgery for prostate cancer, rates of biochemical recurrence are relevant for disease management, patient expectations, and postoperative follow-up. However, while it represents a meaningful outcome of radical prostatectomy, it results from a combination of different factors such as technical skills and/or disease biology, and – when facing a patient with biochemical recurrence – it is often difficult to isolate the relative contribution of each causal factor. For instance, if a patient with undetectable PSA after surgery recurred three years after robot-assisted radical prostatectomy (RARP), it might be challenging to draw definitive conclusions on what exactly led to that recurrence. On the other hand, what is considered by most surgeons as the first and more direct proxy of surgical quality is surgical margins. Surgical margins status is what a surgeon looks for as soon as the pathology report is available, and represents a – modifiable – factor whose appraisal inevitably includes the surgeon’s performance. Moreover, since it is early information after surgery, a surgeon likely remembers specific steps that led to, say, a positive/negative margin, and thus, it is generally easier to relate technical skills with this surgical outcome. In this regard, there is single center evidence that the risk of positive surgical margins after robot-assisted radical prostatectomy (RARP) might be associated with surgeon experience.5 However, this study included only nine surgeons operating at the same institution and as such, might not be entirely generalizable. For this reason, we here sought to investigate whether surgical experience of the surgeons might be associated with the risk of positive surgical margins after anterior robot-assisted radical prostatectomy on a multi-institutional level.
We analyzed data from 14,143 patients treated with robot-assisted radical prostatectomy at ten participating institutions between 2003 and 2022. Patients who received neoadjuvant therapies (n=486) or had missing data for covariates (preoperative PSA, Gleason grade, or stage; n=558) were excluded, leaving 13,090 patients eligible for analysis. Eligible patients were treated by one of 74 surgeons. Surgeons who had previously done robotic radical prostatectomies before their first robotic procedure on a patient in the study cohort were asked to provide details of their previous caseload.
All surgeries were performed with daVinci platforms according to conventional techniques for anterior, trans-peritoneal robot-assisted radical prostatectomy.6 Indication for pelvic lymph node dissection was based on preoperative risk for nodal involvement calculated with the most updated nomogram available at the time of surgery.7 A nerve sparing technique was offered based on patient and cancer characteristics at diagnosis.
Our multivariable model revealed a significant, non-linear association between positive surgical margins and surgical experience (p<0.0001). Figure 1 shows the learning curve for positive margins. The rate of positive surgical margins decreased with increasing surgical experience up to the 2000th procedure, with the steepest decrease in the first 500 procedures. The risk of positive margins for a surgeon with 10, 250, 500, and 2000 prior operations was 26%, 21%, 18%, and 14%, respectively (absolute difference between 10 and 2000 prior procedures: 11%; 95% confidence interval [CI]: 9%, 14%).
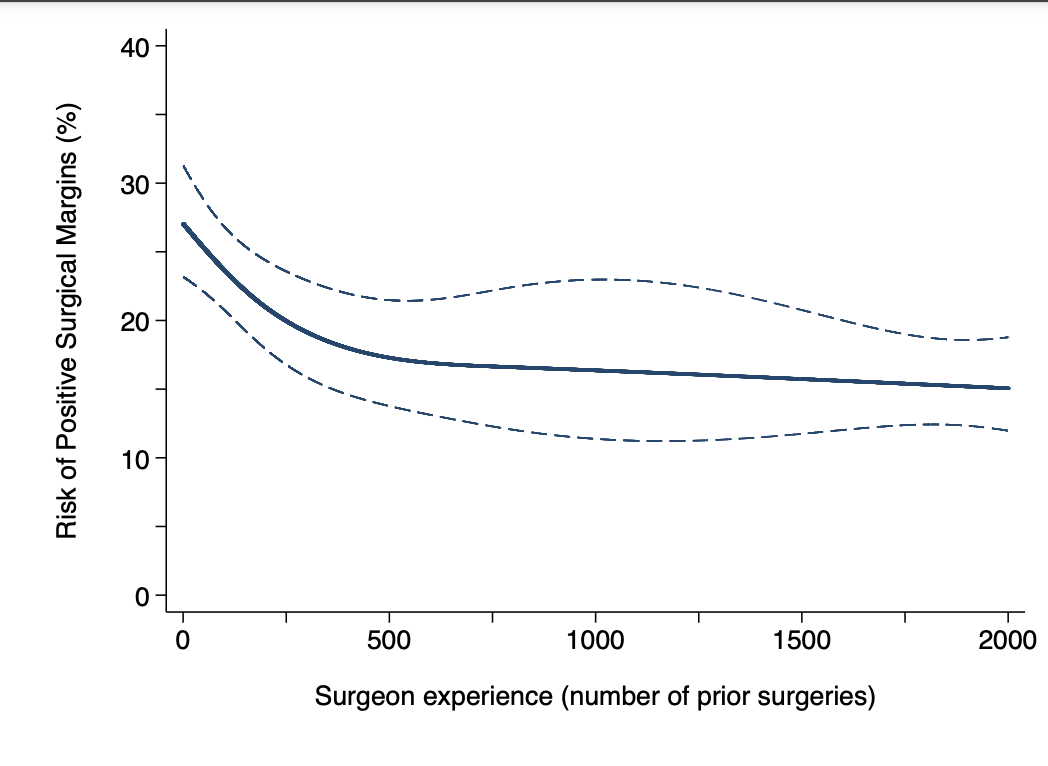
Figure 1. Risk of positive surgical margins for a patient with typical cancer severity over surgical experience (number of prior RARPs). The dotted lines represent 95% confidence interval. RARP: robot-assisted radical prostatectomy.
When stratified by pathological stage T2 vs. T3, our results remained consistent with our main analyses, with a significant, non-linear association between surgical experience and positive surgical margins both in patients with organ-confined (p<0.0001) and extra-prostatic (p=0.005) disease.
In men with pT2 prostate cancer, the risk of positive margins in a patient treated by a surgeon with 10 vs. 250 vs. 500 vs. 2000 prior operations was 22%, 14%, 11%, and 8%, respectively (absolute risk reduction: 13%, 95%CI: 9%, 14%).
Among men with extra-prostatic disease on final pathology, the risk of positive surgical margins was 43%, 38%, 34%, and 30% for patients who were treated by surgeons with 10 vs. 250 vs. 500 vs. 2000 prior robotic prostatectomies, respectively (absolute risk reduction: 13%, 95%CI: 2%, 14%).
Learning curve studies on complex procedures, especially in the field of surgery, raise questions about the mechanism of learning. In line with our results, prior single-surgeon evidence showed that PSM rates plateaued around the 500th procedure.8 However, BCR rates of the same surgeon similarly decreased over time, which contrasts with our previous multi-institutional study.9 Among 8345 patients treated by 46 surgeons, BCR rates of surgeons at the beginning of their learning curve did not differ from those of more experienced ones.2 Although there are substantial differences between the studies – the main one being the single vs. multi-surgeons nature, this evidence opens the discussion on what matters in the field of robot-assisted radical prostatectomy, and it reiterates the debate on the clinical significance of positive surgical margins.10 While it might be hard for a surgeon to remember specific details of the operation if BCR happens long after surgery, info on margin status is available in the early postoperative period. As such, a surgeon is more likely aware of the specific steps that led to a positive margin and thus, he/she might work towards improvement in subsequent operations. On the other hand, the real challenge is to identify what might affect biochemical recurrence regardless of margin status. Since our prior paper did not include patients receiving adjuvant therapies, one reasonable explanation seems patient selection.2 This is consistent with exploratory analyses showing a learning curve for BCR only in subgroups of patients such as those with organ-confined disease.2 Also, it might help explain why the learning curve for BCR after robotic surgery differed from prior findings for open and laparoscopic radical prostatectomy, while the learning curve for surgical margins did not.3,4 Surgeons improved their margin rates over time in all three surgical techniques, but BCR rates followed the same trend only in open and laparoscopic radical prostatectomy. Among possible explanations, improved preoperative risk stratification or imaging modalities (e.g. introduction of MRI for local staging) might result in a better selection of candidates for radical prostatectomy, a phenomenon more likely occurring in more recent robotic operations.
The issue of poor concordance between surgical margins and cancer recurrence warrants further consideration. What seems a compelling similarity in the relationship between these two constructs is the reiterated debate on how ischemia time affects functional outcomes after partial nephrectomy. It is intuitive to assume that longer ischemia might damage the operated kidney; still, this relationship has never been demonstrated as many factors seem involved. Like longer ischemia does not equate to functional deterioration, a positive margin does not invariably expose to recurrence. Therefore, some argue that margin status should not be used to counsel patients after surgery (such as ischemia time rarely is). However, while a longer ischemia might be technically needed in some cases, the same cannot be said for a positive margin. Margin status is more straightforward, binary information is inevitably discussed with the patient as part of the pathology report, and the common claim is that if margins are positive some cancer was left behind. Despite extensive evidence disproving this claim, a positive margin might also have implications in terms of anxiety for patients and/or trigger postoperative treatments with consequent higher healthcare costs.11 Together with a clear need for improved post-operative counseling, these considerations support the argument that, although their relationship with cancer recurrence is debatable, negative margins after surgery are preferable. Under these circumstances, our findings are important for the monitoring of PSM rates and – specifically – for the identification of surgeons above average rates of PSM.12 Our data shows that the same rate of margins has different meanings if analyzed in the context of surgical education. In other words, a given rate of positive margin has different implications for surgeons at different stages of their careers. If, say, a 30% margin rate might be acceptable for a surgeon who did 10 prior cases, it is surely not for a more experienced physician who performed more than 1000 prior RARPs. As a result, we here provide data to establish a reasonable learning curve phase for surgical margins that should plateau not later than a surgeon’s 500th case, also in keeping with prior single-surgeon evidence.8 That said, the final goal of surgical education should be to reach a point where novices achieve the same margin rate as more experienced surgeons since the start of their practice, and future implementations should focus on how to attain this important goal for the whole surgical community.
In conclusion, in contrast with prior evidence that BCR rates after RARP did not differ between experienced and inexperienced surgeons, we found that increasing prior experience was associated with a lower rate of positive margins. While future studies should focus on what more experienced surgeons do to avoid positive margins, further research should explore the relationship between learning, margin rate, and biochemical recurrence. Understanding what margins affect recurrence and whether these margins are trainable or a result of other factors may help explain what is the relative contribution of surgical learning to the achievement of optimal outcomes, and shed light on where to focus future efforts in surgical education.
Written by: Carlo A. Bravi,1,2,3 Paolo Dell’Oglio,4,5,6 Pietro Piazza,7 Simone Scarcella,8,9 Lorenzo Bianchi,7 Ugo Falagario,10 Filippo Turri,11 Iulia Andras,12 Fabrizio Di Maida,13 Ruben De Groote,2,3 Federico Piramide,14 Marcio Covas Moschovas,15 Nazareno Suardi,16,17 Carlo Terrone,16 Giuseppe Carrieri,10 Vipul Patel,15 Riccardo Autorino,18 Francesco Porpiglia,14 Andrew Vickers,19 Alberto Briganti,20 Francesco Montorsi,20 Alexandre Mottrie,2,3 Alessandro Larcher20
- Department of Urology, The Royal Marsden NHS Foundation Trust, London, UK.
- Department of Urology, Onze-Lieve-Vrouwziekenhuis Hospital, Aalst, Belgium
- ORSI Academy, Ghent, Belgium
- Department of Urology, ASST Grande Ospedale Metropolitano Niguarda, Milan, Italy;
- Department of Urology, Antoni van Leeuwenhoek Hospital, The Netherlands Cancer Institute, Amsterdam, The Netherlands
- Interventional Molecular Imaging Laboratory, Department of Radiology, Leiden University Medical Center, Leiden, The Netherlands
- Division of Urology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy
- Division of Urology, United Hospital of Ancona, School of Medicine Marche Polytechnic University, Ancona, Marche, Italy.
- Department of Urology, Azienda Ospedaliera Ospedali Riuniti Marche Nord, Pesaro, Italy.
- Urology and Renal Transplantation Unit, Department of Medical and Surgical Sciences, University of Foggia, Foggia, Italy
- Department of Urology, ASST Santi Paolo e Carlo, University of Milan, Milan, Italy.
- Department of Urology, Iuliu Hatieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania.
- Department of Urology, Careggi University Hospital, Florence, Italy.
- School of Medicine, Division of Urology, Department of Oncology, San Luigi Gonzaga Hospital, University of Turin, Orbassano, Turin, Italy
- AdventHealth Global Robotics Institute, Celebration, FL, USA.
- IRCCS Ospedale Policlinico San Martino, Genova, Italia.
- Department of Urology, Ospedali Civili of Brescia, Brescia, Italy.
- Department of Urology, Rush University, Chicago, IL, USA
- Department of Epidemiology and Biostatistics, Memorial Sloan Kettering Cancer Center, New York, USA.
- Division of Oncology/Unit of Urology; URI; IRCCS Ospedale San Raffaele, Milan, Italy
- Department of Urology, Faculty of Medicine, Medical Centre of the University of Freiburg, Freiburg, Germany
- Department of Urology, Luzerner Kantonsspital, Lucerne, Switzerland
- Department of Urology, Goethe University Hospital Frankfurt, Frankfurt am Main, Germany.
- Department of Urology, ERN eUROGEN Accredited Centre, Ghent University Hospital, Ghent, Belgium.
- Department of Urology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
- Birkmeyer JD. Surgical Skill and Complication Rates after Bariatric Surgery. N Engl J Med. September 2013:1-9.
- Bravi CA, Dell'Oglio P, Mazzone E, et al. The Surgical Learning Curve for Biochemical Recurrence After Robot-Assisted Radical Prostatectomy. European Urology Oncology. July 2022:1-8.
- Vickers AJ, Savage CJ, Hruza M, et al. The surgical learning curve for laparoscopic radical prostatectomy: a retrospective cohort study. Lancet Oncology. 2009;10(5):475-480.
- Vickers AJ, Bianco FJ, Serio AM, et al. The Surgical Learning Curve for Prostate Cancer Control After Radical Prostatectomy. JNCI Journal of the National Cancer Institute. 2007;99(15):1171-1177.
- Bravi CA, Tin A, Vertosick E, et al. The Impact of Experience on the Risk of Surgical Margins and Biochemical Recurrence after Robot-Assisted Radical Prostatectomy: A Learning Curve Study. Journal of Urology. 2019;202(1):108-113.
- Martini A, Falagario UG, Villers A, et al. Contemporary Techniques of Prostate Dissection for Robot-assisted Prostatectomy. European Urology. 2020;78(4):583-591.
- Gandaglia G, Fossati N, Zaffuto E, et al. Development and Internal Validation of a Novel Model to Identify the Candidates for Extended Pelvic Lymph Node Dissection in Prostate Cancer. European Urology. 2017;72(4):1-9. doi:10.1016/j.eururo.2017.03.049.
- Thompson JE, Egger S, Böhm M, et al. Superior Quality of Life and Improved Surgical Margins Are Achievable with Robotic Radical Prostatectomy After a Long Learning Curve: A Prospective Single-surgeon Study of 1552 Consecutive Cases. European Urology. 2014;65(3):521-531.
- Thompson JE, Egger S, Böhm M, et al. Superior Biochemical Recurrence and Long-term Quality-of-life Outcomes Are Achievable with Robotic Radical Prostatectomy After a Long Learning Curve—Updated Analysis of a Prospective Single-surgeon Cohort of 2206 Consecutive Cases. European Urology. 2018;73(5):664-671.
- Vickers A, Bianco F, Cronin A, et al. The Learning Curve for Surgical Margins After Open Radical Prostatectomy: Implications for Margin Status as an Oncological End Point. Journal of Urology. 2010;183(4):1360-1365.
- Martini A, Marqueen KE, Falagario UG, et al. Estimated Costs Associated With Radiation Therapy for Positive Surgical Margins During Radical Prostatectomy. JAMA Netw Open. 2020;3(3):e201913-e201913. doi:10.1001/jamanetworkopen.2020.1913.
- Vickers AJ, Sjoberg D, Basch E, et al. How Do You Know If You Are Any Good? A Surgeon Performance Feedback System for the Outcomes of Radical Prostatectomy. European Urology. 2012;61(2):284-289.


