Since then, multiple large-scale studies have confirmed that AZGP1 loss in prostate cancer strongly predicts clinically poor outcomes, including recurrence, metastasis, and progression to castrate resistance.3,4 Further, AZGP1 predicts survival independent of clinical and pathological parameters such as tumor grade, stage, and pre-operative serum prostate-specific antigen (PSA) level.5-7 Several studies have implicated AZGP1 in immune regulation and key oncogenic signaling pathways in other types of cancer.8, 9 However, the mechanism by which AZGP1 regulates prostate cancer progression remains unclear.
Here, our study revealed that loss of AZGP1 expression promotes angiogenesis in prostate cancer and induces fibrosis in the prostate.10 We first assessed the expression levels of AZGP1 in different prostate cancer cell lines and observed that both transcript and protein levels of AZGP1 were significantly higher in androgen receptor (AR)-positive cell lines compared to AR-negative ones. Subsequently, we modulated AZGP1 expression levels by either underexpressing or overexpressing it in prostate cancer cells. However, knockout of AZGP1 in prostate cancer cells did not alter their proliferation, clonal growth, migration, or invasion in vitro.
Later, we utilized a mouse model in which the AZGP1 gene had been inactivated (AZGP1-/-mice) to investigate its role in the prostate. The normal prostate in the AZGP1-/- mice had a significant increase in blood vessel density within the mesenchyme of prostates from 6-month-old and 10-month-old AZGP1-/- mice (Figure 1).

Notably, there were no significant differences in the epithelial anatomy of the dorsal and lateral prostate lobes between wild-type and AZGP1-/- mice. Additionally, we observed increased fibrosis and collagen deposition, confirmed by Sirius red staining, near the base of the seminal vesicles in the prostates of 6-month-old and 10-month-old AZGP1-/- mice.
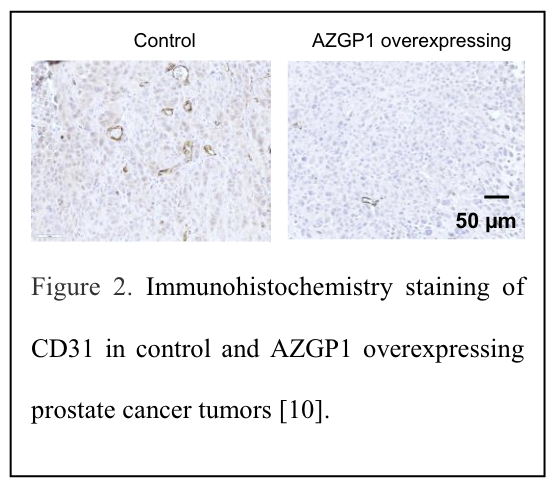
To deepen our understanding of AZGP1's regulation of angiogenesis in prostate cancer, we conducted in vivo studies using AZGP1-overexpressing human prostate cancer cells. Consistently, we observed no changes in tumor weight or volume in AZGP1- overexpressing xenografts. However, we did notice an increased number of small blood vessels, confirmed by immunohistochemistry staining, in these tumors (Figure 2).
To delve further, we performed mass spectrometry-based proteomic analysis using tumor tissues from both control and AZGP1-overexpressing xenografts. Remarkably, we identified a significant overlap of proteins associated with the angiogenesis pathway with AZGP1-regulated proteins. In particular, western blot analysis confirmed the downregulation of MMP-9 and the upregulation of PDCD6 and EphA2 in AZGP1-overexpressing tumors.
To directly assess the impact of AZGP1 on angiogenesis, we conducted in vitro angiogenesis assays using human umbilical vein endothelial cells (HUVECs). Our findings revealed that AZGP1 treatment significantly suppressed HUVEC growth and migration as well as their ability to form vessel-like structures in vitro.
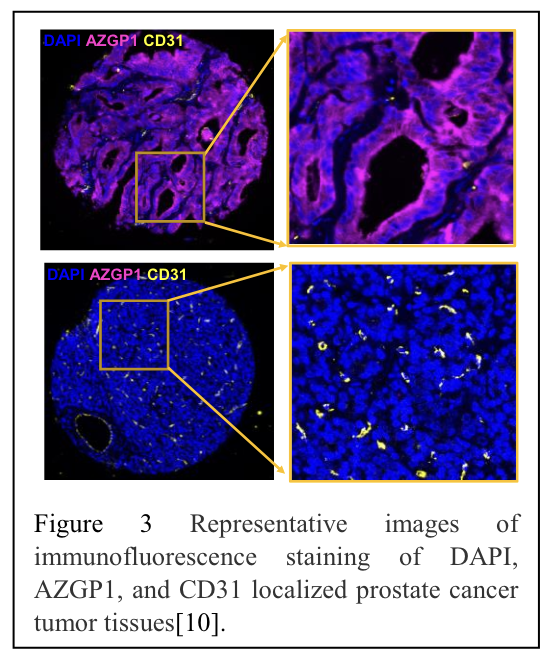
Furthermore, we validated our results in human prostate cancer specimens. Employing a deep-learning model, we identified blood vessels based on a combination of CD31, CD34, and collagen IV staining.11 Our analysis demonstrated an inverse correlation between AZGP1 expression and microvessel density in a human tissue microarray of localized prostate cancer (Figure 3).
In summary, our study reveals that AZGP1 exerts a negative regulatory effect on angiogenesis in prostate cancer. AZGP1 loss promotes fibrosis and angiogenesis in the normal prostate in an AZGP1 knockout mouse model. AZGP1 inhibits angiogenesis in prostate cell line xenograft models and human tumor tissues. Our findings suggest that in addition to being an important biomarker in prostate cancer, AZGP1 could serve as a promising therapeutic target for prostate cancer.
Funding Support: National Institutes of Health and Department of Defense.
Written by: Ru M Wen,1 Hongjuan Zhao,1 and James D Brooks1,2
- Department of Urology, Stanford University School of Medicine, Stanford, CA 94305, USA
- Canary Center at Stanford for Cancer Early Detection, Stanford University School of Medicine, Stanford, CA 94305, USA
- Siegel, R.L., et al., Cancer statistics, 2023. CA Cancer J Clin, 2023. 73(1): p. 17-48.
- Lapointe, J., et al., Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci U S A, 2004. 101(3): p. 811-816.
- Brooks, J.D., et al., Loss of expression of AZGP1 is associated with worse clinical outcomes in a multi‐institutional radical prostatectomy cohort. Prostate, 2016. 76(15): p.1409-1419.
- Winther, M.D., et al., AZGP1 Protein Expression in Hormone-Naïve Advanced Prostate Cancer Treated with Primary Androgen Deprivation Therapy. Diagnostics (Basel), 2020. 10(8).
- Kristensen, G., et al., Predictive value of AZGP1 following radical prostatectomy for prostate cancer: a cohort study and meta-analysis. J Clin Pathol, 2019: p. jclinpath-2019-205940.
- Severi, G., et al., A three-protein biomarker panel assessed in diagnostic tissue predicts death from prostate cancer for men with localized disease. Cancer Med, 2014. 3(5): p.1266-74.
- Yip, P.Y., et al., Low AZGP1 expression predicts recurrence in margin‐positive, localized prostate cancer. The Prostate, 2011. 71(15): p. 1638-1645.
- Hassan, M.I., et al., Zinc alpha 2-glycoprotein: a multidisciplinary protein. Mol Cancer Res, 2008. 6(6): p. 892-906.
- Tian, H., et al., Downregulation of AZGP1 by Ikaros and histone deacetylase promotes tumor progression through the PTEN/Akt and CD44s pathways in hepatocellular carcinoma. Carcinogenesis, 2017. 38(2): p. 207-217.
- Wen, R.M., et al., AZGP1 deficiency promotes angiogenesis in prostate cancer. Journal of Translational Medicine, 2024. 22(1): p. 383.
- Karageorgos, G.M., et al., Deep learning-based automated pipeline for blood vessel detection and distribution analysis in multiplexed prostate cancer images. Front Bioinform, 2023. 3: p. 1296667.


