Although multiparametric magnetic resonance imaging (MRI) is recommended before biopsies, many unnecessary procedures persist despite improvements in detecting significant cancer (csPCa). While the "Schoots model," which combines PSAd and MRI findings to guide biopsy decisions, is mentioned in the recent European Association of Urology guidelines, its effectiveness is hindered by a lack of validation and standardization.
The study aims to assess the risk of significant cancer using various imaging methods for prostate volume estimation and evaluate the performance of the Schoots model in a large European patient group.
We analyzed data from 4841 MRI-targeted biopsy-naïve patients with clinically localized PCa between January 2016 and April 2023. Out of these, 971 patients were included, meeting the criteria for complete information on clinical, radiological, and biopsy data.
Prebiopsy MRIs were conducted following European Society of Urogenital Radiology guidelines, using 1.5-T or 3-T scanners, with or without an endorectal coil. Suspicious lesions were identified based on PI-RADS scores and submitted to biopsy. Prostate biopsies were performed using the KOELIS® system, with a minimum of 3 cores per target taken combined with bilateral systematic biopsies.
Tridimensional-US (3D-US) volume was obtained through transrectal US semi-automated segmentation, while MRI volume was calculated using ellipsoidal formula with 3-axis measurements. Each patient was assigned a risk category based on the risk-adapted table by Schoots (Figure 1).
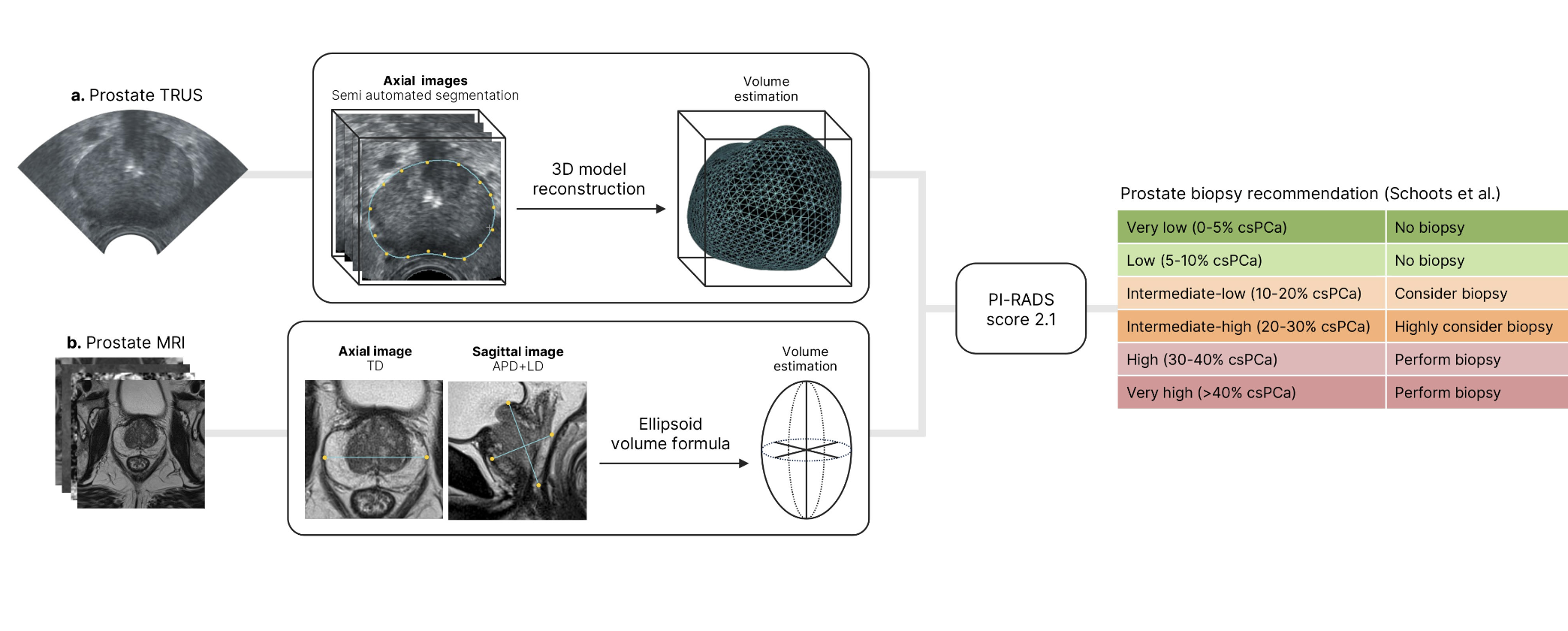
The primary outcome assessed the distribution of csPCa based on imaging modality in the Schoots risk-adapted table, while the secondary outcome evaluated the model's performance in predicting csPCa risk.
The patients' characteristics revealed a median pre-biopsy PSA value of 7.3 ng/ml, with median prostate volumes of 49 cc using 3D-US and 47 cc using MRI. Median PSAd was 0.14 ng/ml/cc with 3D-US and 0.15 ng/ml/cc with MRI. PI-RADS scores for the index MRI lesion ranged from 2 to 5, with 3.8% of patients showing positive digital rectal examination and a csPCa detection rate of 43%.
In comparing prostate volume measurements, 3D-US yielded significantly higher median volumes compared to MRI across quartiles. While there was a significant correlation between the two modalities, notable differences were observed, especially for larger prostates.
The distribution of csPCa patients varied based on PI-RADS score and PSAd according to the Schoots model. External validation showed a slightly higher accuracy for the Schoots model when using 3D-US compared to MRI, though not statistically significant. Calibration plots demonstrated satisfactory graphical analysis, with a tendency towards over-estimation. Decision curve analysis demonstrated an improvement of net benefit using 3D-US for probability thresholds above 25%. Notably, in patients with the largest prostates, the model's performance remained consistent between 3D-US and MRI.
There are several limitations that must be underlined. Retrospective analysis poses a risk of selection bias, and the relatively low proportion of patients with PI-RADS score 2 may limit generalizability. Furthermore, the involvement of experienced referral centers with dedicated physicians at each procedural step may restrict broader applicability. Surgical pathology data for patients undergoing radical prostatectomy were lacking.
In conclusion, the study suggests that prostate volume estimation through semi-automated segmentation with the KOELIS® system should be favored over the ellipsoidal formula for estimating PSAd, leading to improved risk stratification for predicting csPCa.
Written by: Romain Diamand, MD, PhD & Arthur Baudewyns, MD
Department of Urology, Jules Bordet Institute-Erasme Hospital, Hôpital Universitaire de Bruxelles, Université Libre de Bruxelles, Brussels, Belgium
Read the Abstract


