Advances in diagnostic imaging with multiparametric magnetic resonance imaging (MRI) have shown promising results in the identification of focal lesions that alongside technological advancements in high-precision imaging-guided radiotherapy (IGRT), have enabled the safe and efficient implementation of dose escalation procedures in routine clinical practice Given the known dose-response relationship in the radiotherapy treatment of PCa, focal dose escalation restricted to the dominant lesion (DL) would hypothetically lead to better local disease control without significantly increasing urinary or rectal toxicity.
The clinical benefits and tolerance of focal boosts to the DL have been previously reported. Several studies confirm that MRI-guided focal dose intensification is associated with excellent biological, anatomical, and functional responses with acceptable toxicity and good quality of life.1-3
In the last decade, coinciding with the clinical implementation of high-precision and efficient technological advances, there has been a growing interest in the use of extreme or ultra-hypofractionation radiotherapy (UHRT) using stereotactic ablative (SBRT-SABR) techniques. SABR is a form of real-or near-real-time IGRT that uses high doses of radiation delivered in a precise and targeted method to the tumour in a very limited number of fractions. The reduction in the number of fractions, although it involves more complex technology and consumes more time per fraction, is an important advance for the quality of life of patients, by reducing their number of hospital visits.
SABR is a form of real-time or near-real-time image-guided radiotherapy (IGRT) that delivers high doses of radiation in a precise and targeted manner to the tumour over a very limited number of fractions. Although this reduction in the number of fractions involves more complex technology and requires more time per session, it significantly improves patients' quality of life by reducing the number of hospital visits.
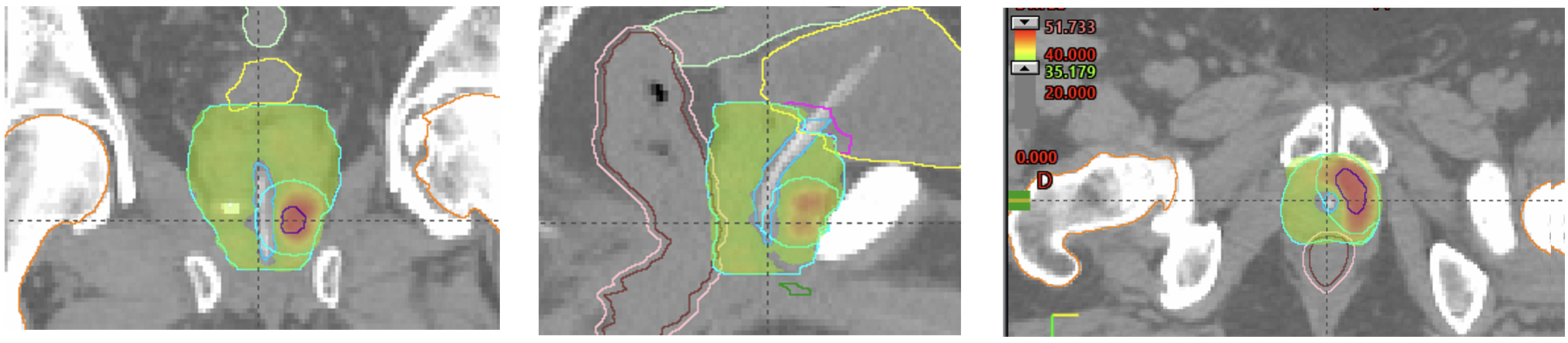
In this context, the integration of SABR with imaging-guided focal dose intensification represents a promising treatment approach that has been reported to be feasible and safe but at potential risk of increased toxicity, mainly urinary. Implementing dose minimization protocols for adjacent organs, specifically the urethra, bladder trigone, and rectum, would reduce the incidence of associated complications.
Most of the evidence for UHRT has been reported in low and intermediate-risk PCa, while there is very limited data available in high-risk disease,4 a scenario where dose intensification has particularly proven to improve local control.
The SAFO phase II trial has been designed to evaluate the effectiveness, safety, and impact on the quality of life of MRI-guided focal dose intensification using SABR technology and dose minimization of the urethra and bladder trigone, in intermediate and high-risk PCa patients. This trial also includes a translational study of immuno-profiling in the population of high-risk disease.
The hypothesis of this study is that focal dose intensification using SABR-MRI guided techniques with urethra/bladder trigone sparing would lead to a higher probability of local control without a significant increase in toxicity compared to standard clinical practice. The results will help determine the design of subsequent based phase III trials in the SABR setting for high-risk disease, the value of MRI in monitoring response, and hopefully the impact of immune phenotyping in the individualized approach of localized PCa.
In this scenario of high-risk disease where the treatment approach is fraught with numerous unresolved issues—mainly the selection of patients for either intensification or de-escalation of treatment- the improvement in local control while preserving quality of life is of paramount importance. With this in mind, our next research focus is on MRI radiomics as a potential biomarker for outcomes, toxicity, and monitoring in patients undergoing radiotherapy for localized PCa
Written by: Almudena Zapatero MD, PhD, Health Research Institute, University Hospital La Princesa, Madrid, Spain
References:
- Kerkmeijer LGW, Groen VH, Pos FJ, et al. Focal boost to the intrapro-static tumor in external beam radiotherapy for patients with localized prostate cancer: results from the FLAME randomized phase III trial. J Clin Oncol. 2021;39:787-96.
- Zapatero A, Roch M, Castro Tejero P, Büchser D, Martin de C, Vidales S, González, et al. MRI-guided focal boost to dominant intraprostatic lesion using volumetric modulated arc therapy in prostate cancer. Results of a phase II trial. Br J Radiol. 2022;95:20210683.
- Darren MC, Poon J, Yuan B, Yang, Linda GW, Kerkmeijer d, Amar U. Kishan E, Vedang Murthy f, Alison Tree g, Almudena Zapatero h, Oi Lei Wong. Magnetic resonance imaging-guided focal Boost to Intraprostatic lesions using External Beam Radiotherapy for localized prostate Cancer: a systematic review and Meta-analysis. Eur Urol Oncol. 2022. https://doi.org/10.1016/j. euo.2022.10.001.
- van Ritchell NY, Jiang DB, Fuller A, Loblaw T, Jiang AJ, Katz et al. Stereotactic body radiotherapy for high-risk localized Carcinoma of the prostate (SHARP) Consortium: analysis of 344 prospectively treated patients, International. J Radiation Oncology*Biology*Physics Volume 110, Issue 3,2021, Pages 731- 737, ISSN 0360-3016, https://doi.org/10.1016/j.ijrobp.2021.01.016.


