177Lu-PSMA-617 is approved for the treatment (tx) of mCRPC. Though tx is associated with improved survival, not all pts experience a benefit. Acquired resistance is common and some pts have intrinsic resistance. There is a lack of data on genomic markers that could aid in selecting pts for tx. In this study, we aim to characterize molecular predictors of benefit to 177Lu-PSMA-617.
Methods:
We used the retrospective Prostate Cancer Precision Medicine Multi-Institutional Collaborative Effort (PROMISE) clinical-genomic database (n=2100). The primary endpoint was to investigate the association of genomic alterations with a ≥50% PSA decline (PSA50) from baseline following 177Lu-PSMA-617. Associations were assessed using Wald-chi square test and Cox regression in multivariable analysis. Secondary endpoints included clinical progression-free survival (PFS) and overall survival (OS).
Results:
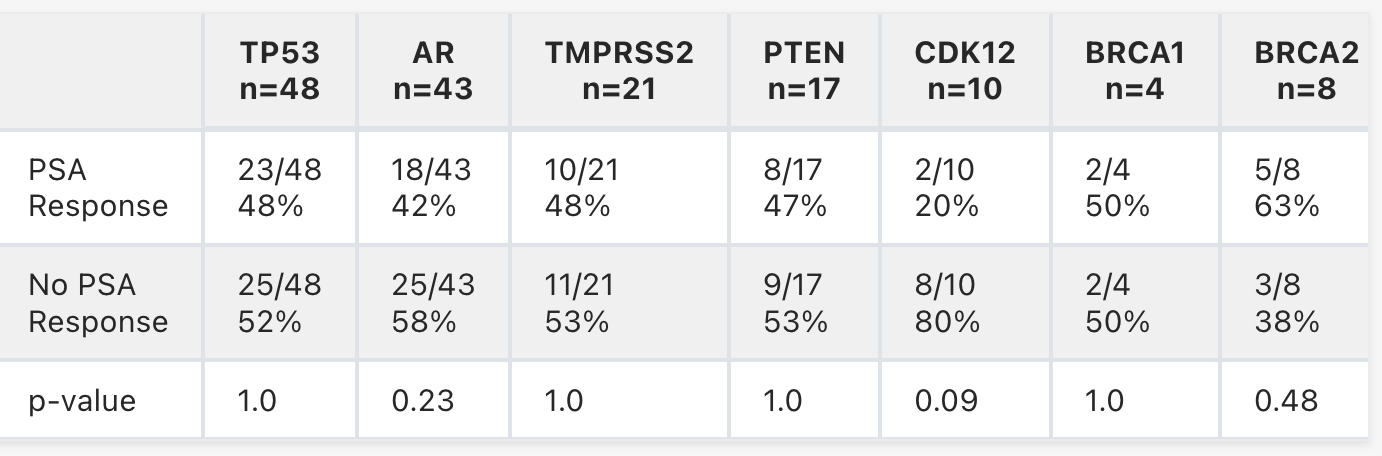
We identified 115 pts with PSMA PET+ mCRPC treated with 177Lu-PSMA-617 who had commercial genetic sequencing prior to tx (median age 72 yrs, 25% non-white). Median number of prior lines for mCRPC was 3 with 71 pts (62%) receiving >1 androgen receptor signaling inhibitor (ARSI) and 55 pts (48%) receiving >1 taxane; 11 pts (9%) received ARSI with 177Lu-PSMA-617. Overall, the PSA50 was 49% with median OS and PFS of 14.0 and 7.6 months (mos), respectively. In pts with a PSA50, median OS and PFS were 22.6 and 11.6 mos, respectively, vs 11.2 and 5.6 mos for those without a PSA50. Genetic alterations associated with PSA50 are in the table. PSA50 was 48% in pts with (n=32) vs 49% in pts without DDR alterations (n=83). PSA50 was 44% in pts with tumor suppressor gene alterations (TSGa) (PTEN, p53, RB1) (n=68) vs 56% in pts without (n=47). Median PFS was 7.6 vs 7.3 mos for pts with and without any TSGa (p=0.90), and median OS was 12.2 mos vs 22.6 mos for pts with and without TSGa (p=0.004). Of the 43 pts with AR alterations, 8/15 (53%) with LBD mutations, 10/27 (37%) with AR amplification, and 0/1 with AR-V7 had a PSA50. FGFR, CDK12 and MYC alterations were enriched in individuals without a PSA50 (75-83%).
Conclusions:
We demonstrate that CDK12, MYC and FGFR alterations were associated with a lower PSA50 with 177Lu-PSMA-617. Larger cohorts should be investigated for confirmation, as biomarkers to inform relative benefit of tx could be useful in prioritizing options for mCRPC.
- Justine Panian, University of California, San Diego, San Diego, CA
- Nicholas Henderson, Department of Biostatistics, University of Michigan, Ann Arbor, MI
- Pedro C. Barata, University Hospitals Seidman Cancer Center, Cleveland, OH
- Mehmet Asim Bilen, Winship Cancer Institute of Emory University, Atlanta, GA
- Laura Graham, University of Colorado Cancer Center Anschutz Medical Campus, Aurora, CO
- Elisabeth I. Heath, Karmanos Cancer Institute, Wayne State University, Detroit, MI
- Daniel Herchenhorn, Oncologia D'Or, Rio De Janeiro, Brazil
- Clara Hwang, Division of Hematology/Oncology, Department of Internal Medicine, Henry Ford Cancer, Detroit, MI
- Deepak Kilari, Department of Medicine, Division of Hematology and Oncology, The Medical College of Wisconsin, Milwaukee, WI
- Vadim S Koshkin, Division of Hematology and Oncology, Department of Medicine, University of California, San Francisco, San Francisco, CA
- Jones T. Nauseef, Division of Hematology & Medical Oncology, Weill Cornell Medicine; Sandra and Edward Meyer Cancer Center, New York, NY
- Alexandra Sokolova, OHSU Knight Cancer Institute, Portland, OR
- Yousef Zakharia, University of Iowa Holden Comprehensive Cancer Center, Iowa City, IA
- Michael Thomas Schweizer, Division of Hematology & Oncology, University of Washington & Fred Hutchinson Cancer Center, Seattle, WA
- Tanya B. Dorff, City of Hope Comprehensive Cancer Center, Duarte, CA
- Andrew J. Armstrong, Division of Medical Oncology, Duke University Medical Center, Duke Cancer Institute Center, Durham, NC
- Ajjai Shivaram Alva, Department of Internal Medicine, Division of Hematology and Oncology, University of Michigan, Ann Arbor, MI
- Rana R. McKay, University of California, San Diego Health, La Jolla, CA


