We performed a large study (IP5-MATTER) of 300 patients across multiple centres in the United Kingdom to ascertain how patients diagnosed at their first diagnosis with advanced (metastatic) prostate cancer made decisions regarding additional offered treatments (surgery, radiotherapy, ablation) for their prostate and cancer deposits (specialised “SABR” radiotherapy), respectively.
These additional treatments would not provide cure but may reduce cancer burden (cytoreduction), prolong life, and extend time without cancer progression. We used a specialised health economic model called a “discrete choice experiment”. A discrete choice experiment instrument allows researchers to understand how patients trade-off between the treatment attributes. The trade-off in our study was whether patients were willing to accept the likely increased toxicity with additional treatment(s) in exchange for potential extended months of life or months without their cancer progressing further.
We found that most patients were willing to accept these additional treatments for survival benefits, in particular treatments that preserved urinary function and reduced fatigue. We were able to calculate the exact trade off value for a given side effect, this can be used to calculate whether most patients would be accepting of the benefit from additional treatments currently available and those that may be reported in ongoing clinical trials.
Academic Summary
Background: Cytoreductive treatments for patients diagnosed with de novo synchronous metastatic hormone-sensitive prostate cancer (mHSPC) confer incremental survival benefits over systemic therapy, but these may lead to added toxicity and morbidity.1-3
Recent advances in standard systemic therapy, beyond androgen deprivation therapy (ADT), have improved reported overall survival (OS) from a median of 44 to 63 months.4,5 This has created an oncological “window of opportunity” to explore the benefit of cytoreductive treatments to the residual primary tumour and established metastases, in an attempt to achieve further disease control.1,5
However, we understand very little about patients’ preferences and decision-making in relation to the application of novel cytoreductive treatments with noncurative intent that may lead to substantial toxicity and morbidity for this patient group.7
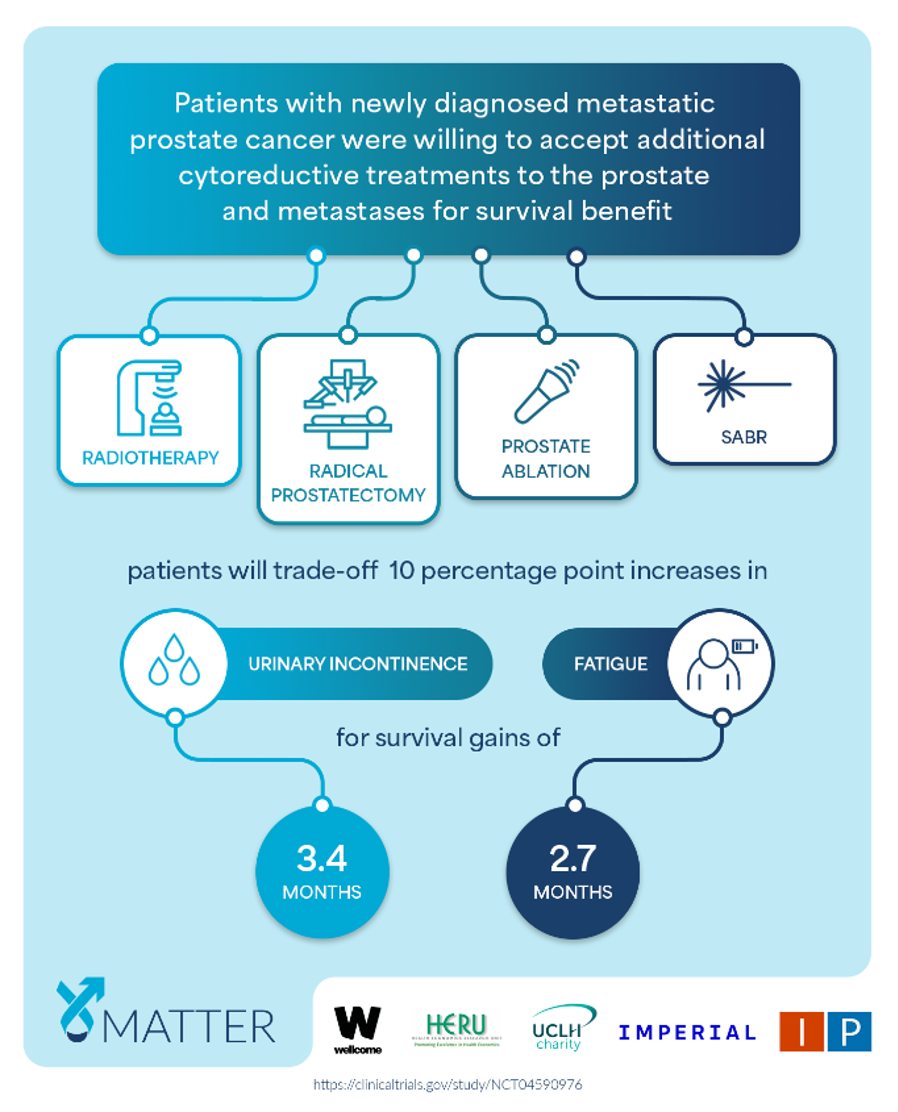
Our objective was to determine patients’ preferences for, and trade-offs between, additional cytoreductive prostate and metastasis-directed interventions.
Methods: A prospective multicentre discrete choice experiment trial was conducted at 30 hospitals in the UK between December 3, 2020, and January 25, 2023 (NCT04590976). The individuals were eligible for inclusion if they were diagnosed with de novo synchronous mHSPC within 4 months of commencing androgen deprivation therapy and had performance status 0–2. A discrete choice experiment instrument was developed to elicit patients’ preferences for cytoreductive prostate radiotherapy, prostatectomy, prostate ablation, and stereotactic ablative body radiotherapy to metastasis. Patients chose their preferred treatment based on seven attributes. An error-component conditional logit model was used to estimate the preferences for and trade-offs between treatment attributes.
Key findings: A total of 352 patients were enrolled, of whom 303 completed the study. The median age was 70 yr (interquartile range [IQR] 64–76) and prostate-specific antigen was 94 ng/ml (IQR 28–370). Metastatic stages were M1a 10.9% (33/303), M1b 79.9% (242/303), and M1c 7.6% (23/303). Patients preferred treatments with longer survival and progression-free periods. Patients were less likely to favour cytoreductive prostatectomy with systemic therapy (Coef. –0.448; [95% confidence interval {CI} –0.60 to –0.29]; p < 0.001), unless combined with metastasis-directed therapy. Cytoreductive prostate radiotherapy or ablation with systemic therapy, number of hospital visits, use of a “day-case” procedure, or addition of stereotactic ablative body radiotherapy did not impact treatment choice.
Patients were willing to accept an additional cytoreductive treatment with 10 percentage point increases in the risk of urinary incontinence and fatigue to gain 3.4 months (95% CI 2.8–4.3) and 2.7 months (95% CI 2.3–3.1) of overall survival, respectively.
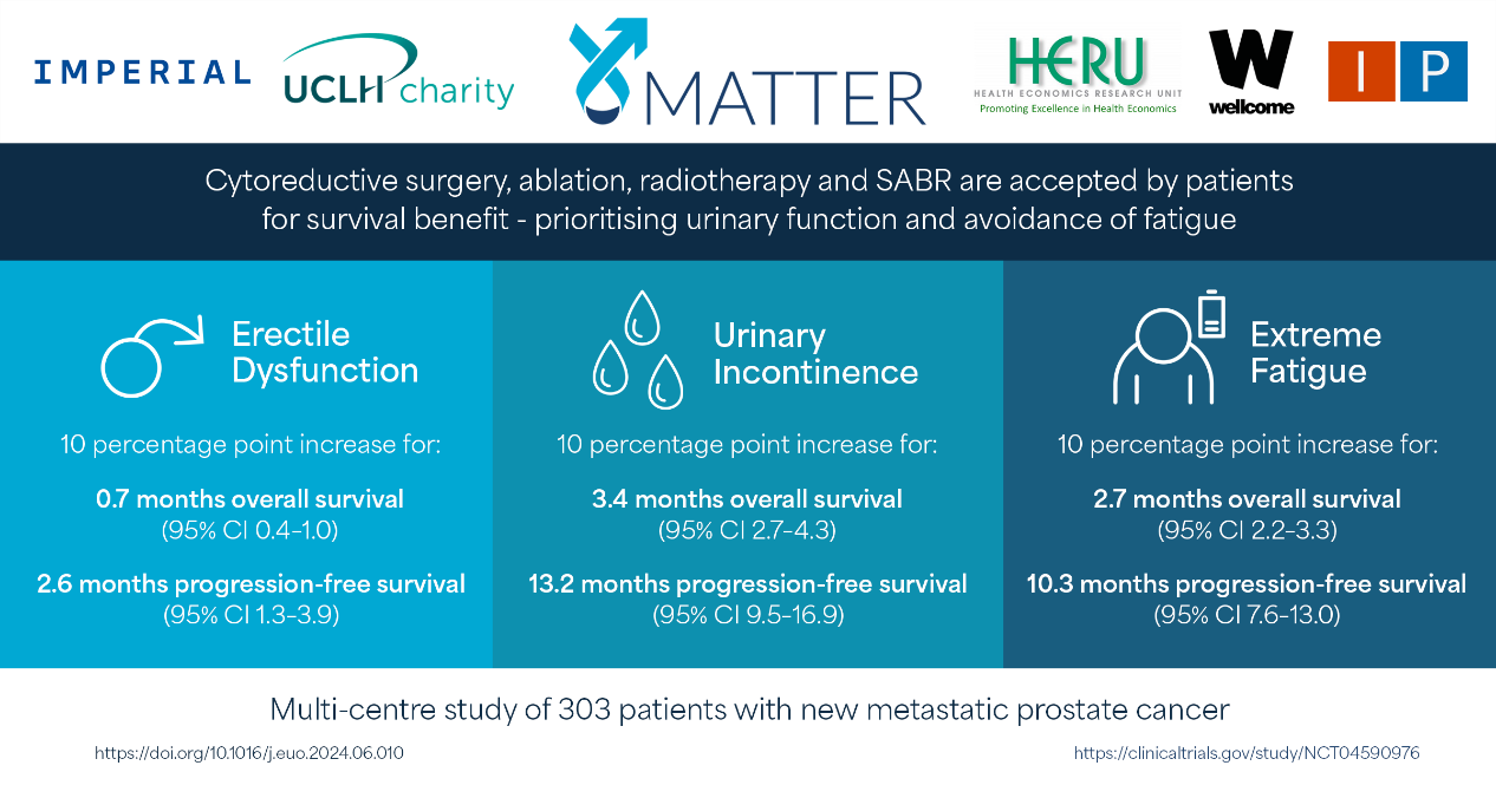
Conclusions: To our knowledge, IP5-MATTER is the first study to ascertain patients’ preferences and trade-offs for additional cytoreductive treatments in de novo synchronous mHSPC.
There is growing evidence that decisions made by patients in discrete choice experiments are reflective of real-world healthcare decisions. A strength of our study is that participants’ baseline characteristics, Gleason/ISUP grade group, tumour and nodal staging, PSA, and metastatic burden were all directly comparable with those reported in the STAMPEDE (Arm-H) randomised study that confirmed the benefit of cytoreductive prostate radiotherapy.2 Thus, our study results suggest that the median survival benefit (63.6 vs 85.5 mo; standard of care [SOC] vs SOC with cytoreductive radiotherapy in low burden) and an adverse event profile for cytoreductive prostate radiotherapy are likely to be accepted by most patients.
Discrete choice experiments assessing patients’ perspectives play an important role in regulatory approval of new treatments and health technologies. The US Food and Drug Administration guidance cites discrete choice experiments as its favoured stated preference elicitation approach.8-10 Following the STAMPEDE (Arm-H) study, regulatory approval for additional cytoreductive prostate radiotherapy was agreed in Europe, but approval remains elusive elsewhere. IP5-MATTER provides additional information to promote the commissioning of cytoreductive radiotherapy.
Finally, this study can be used to determine the likely acceptance of the effect sizes due to being reported in currently recruiting multimodal cytoreductive treatment trials (eg, IP2-ATLANTA and SWOG 1802).11-12 These current trials combine surgery, ablation, radiotherapy, and/or stereotactic ablative radiotherapy.
In summary, the IP5-MATTER study reported patients are accepting additional cytoreductive treatments for survival benefits in mHSPC, prioritising the preservation of urinary function and avoidance of fatigue.
*Joint collaboration between Imperial College London and the Health Economics Research Unit (HERU), University of Aberdeen. Charity-funded academic study by the Wellcome Trust (204998/Z/16/Z) & University College London Hospitals Charity (P83624/1348). Prospectively registered on ClinicalTrials.gov, number NCT04590976.
Written by: Martin J. Connor PhD, FHEA, MRCS, MSc, BSc (Hons), MBBS, NIHR Clinical Lecturer in Uro-Oncology, Imperial College London, London, England
References:
- M.J. Connor. Cytoreductive treatment strategies for de novo metastatic prostate cancer. Nature Reviews Clinical Oncology 17, no. 3 (2020): 168-182.
- C.C. Parker, N.D. James, C.D. Brawley, et al. Radiotherapy to the prostate for men with metastatic prostate cancer in the UK and Switzerland: long-term results from the STAMPEDE randomised controlled trial PLoS Med, 19 (2022)
- M.J. Connor. Targeting oligometastasis with stereotactic ablative radiation therapy or surgery in metastatic hormone-sensitive prostate cancer: a systematic review of prospective clinical trials. European Urology Oncology 3, no. 5 (2020): 582-593.
- C.L. Vale, D.J. Fisher, I.R. White, et al. What is the optimal systemic treatment of men with metastatic, hormone-naive prostate cancer? A STOPCAP systematic review and network meta-analysis Ann Oncol, 29 (2018), pp. 1249-1257
- C.J. Sweeney, Y. Chen, M. Carducci, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer N Engl J Med, 373 (2015), pp. 737-746
- R.R. Weichselbaum, S. Hellman Oligometastases revisited Nat Rev Clin Oncol, 8 (2011), p. 378
- M.J. Connor, V. Khoo, V. Watson, H.U. Ahmed Radical treatment without cure: Decision-making in oligometastatic prostate cancer Eur Urol, 79 (2021), pp. 558-560
- US Food and Drug Administration. Patient preference information–voluntary submission, review in premarket approval applications, humanitarian device exemption applications, and de novo requests, and inclusion in decision summaries and device labeling. Guidance for industry, Food and Drug Administration staff, and other stakeholders. 2016; 2017.
- A.C. Mühlbacher, C. Juhnke, A.R. Beyer, S. Garner Patient-focused benefit-risk analysis to inform regulatory decisions: The European Union perspective Value Health, 19 (2016), pp. 734-740
- M. Ho, A. Saha, K.K. McCleary, et al. A framework for incorporating patient preferences regarding benefits and risks into regulatory assessment of medical technologies Value Health, 19 (2016), pp. 746-750
- ClinicalTrials.gov. Standard systemic therapy with or without definitive treatment in treating participants with metastatic prostate cancer (SWOG 1802). NCT03678025. https://clinicaltrials.gov/ct2/show/NCT03678025
- M.J. Connor, T.T. Shah, K. Smigielska, et al. Additional treatments to the local tumour for metastatic prostate cancer-assessment of novel treatment algorithms (IP2-ATLANTA): Protocol for a multicentre, phase II randomised controlled trial BMJ Open, 11 (2021), Article e042953


